calsfoundation@cals.org
Rotifers
aka: Wheel Animals
The Phylum Rotifera (“wheel animals”) contains over 2,100 nominal taxa of microscopic and near microscopic species of unsegmented, bilaterally symmetrical pseudocoelomate invertebrates. They were originally named in 1696 by Anglican priest John Harris (1666–1719) and studied in 1703 by Antoine van Leeuwenhoek (1632–1723). Several surveys of Rotiferans have been done in Arkansas, although there is no summation of the species as of 2019.
Because they are minute and mostly composed of soft bodies, rotifers are not commonly supported for fossilization. Their only hard parts, their jaws, are sometimes preserved in the fossil record, but their size makes detection challenging. However, fossils of Habrotrocha angusticollis have been found in Pleistocene (2.5 million to 11,700 years ago) peat deposits of Ontario, Canada. The oldest reported fossil rotifers have been found in Dominican amber dating to the Eocene Epoch (56 to 33.9 million years ago).
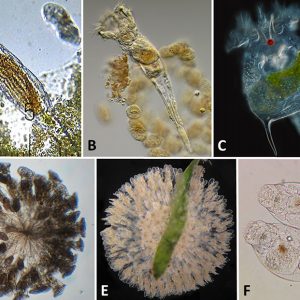
Rotifers are among the most conspicuous, highly diverse freshwater micro-metazoans (less than ten percent are marine) that occupy keystone positions in aquatic ecosystems. Most range in size from 200 to 500 micrometers (µm) to over 2 mm (0.08 in.) such as Rotaria neptunia. Rotifers resemble protozoans and gastrotriches; however, those groups do not have jaws, and their ciliation is not distributed in the same way as rotifers’. Rotifers generally are free-swimming and thus planktonic; however, there are about twenty-five colonial species and even some species that move by inching along a substrate. Rotifers are widely distributed mostly in freshwater habitats across the world, with some endemic such as Cephalodella vittata in Lake Baikal, Russia.
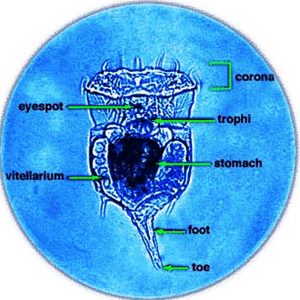
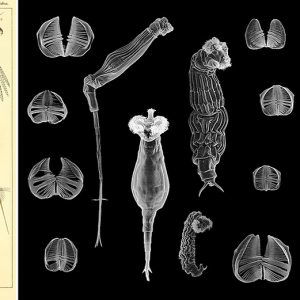
Rotifers are multicellular organisms (about 1,000 cells) with body cavities that are partially lined by mesoderm. These invertebrates have specialized organ systems and a complete digestive tract that includes both a mouth and an anus. Morphologically, rotifers have an integument (body wall with cuticle) that contains a filament layer of varying thickness. Internally, rotifers possess a perivisceral cavity called a pseudocoelom that bathes its muscles and its nerve, digestive, reproductive, and protonephridial organs. Rotifers also have two conspicuous features: (1) a specialized ciliated organ called the corona that caps the crown in the buccal field and (2) a muscular pharynx (mastax) that includes a complex set of jaws called trophi. These jaws are taxonomically unique, allowing scientists to distinguish species by characteristic features of these petite anatomical structures. Rotifers are bilaterally symmetrical, possess a cuticle that covers all of the body, and have a variety of shapes from cylindrical to saccate, with some even worm-like in appearance. Characteristically, they are divided into three or four regions: the head (corona), neck (in some forms), body (trunk), and foot, which may be absent in planktonic forms. These three or four regions are not actually segments but are instead folds in the body that function like joints or allow the body to collapse telescopically. The foot may possess pedal glands that secrete an adhesive for temporary attachment to a surface.
The classification of rotifers is in a state of flux in the twenty-first century, and their taxonomy is currently under review. Some suggest there are two classes of rotifers including the Class Pararotatoria (containing one family, Seisonidae) and Class Eurotatoria, which is made up of two subclasses (Bdelloidea and Monogonata). However, others feel there are three classes: Seisonidea (one genus, three species), Bdelloidea (about 590 species), and Monogononta (about 1,400 species). In some recent treatments, rotifers are placed with acanthocephalans (Phylum Acanthocephala) in a larger clade called the Syndermata.
In general, rotifers are solitary free-swimming invertebrates. They may function as potential prey or mates; however, a number of species within ten genera form intraspecific colonies or join in the formation of interspecific colonies. The colonial species belong to two primary families (Conochilidae and Flusculariidae); however, colonial formation has been discovered in at least one bdelloid rotifer. Colonies range from fewer than five individuals to more than 500 individuals as in the free-swimming colonial flosculariid rotifer, Lacinularia elliptica. Sessile rotifer species are commonly found in ponds and lakes, including Atrochidae (two genera) and Flosculariidae (nine genera).
Within the Rotifera, reproduction types vary broadly. In the Class Seisonidea, they reproduce through bisexual means (gametogenesis occurring via classical meiosis with the production of two polar bodies) with fully functional males and females equally common in a population. However, in the Class Bdelloidea, species reproduce entirely by asexual parthenogenesis; thus, only females are present. In the Class Monogononta, smaller males appear only sporadically. In males, a single testis produces sperm that make their way through a ciliated vas deferens to a gonopore. Males also have an eversible penis that injects sperm into the female’s pseudocoelom. Most females have a single ovary and a vitellarium that produces yolk for the eggs. Following fertilization, eggs travel through an oviduct and out the opening of the cloacal bladder. Rotifers either produce amictic eggs (thin-shelled diploid summer eggs that develop into amictic females) or mictic eggs (thin-shelled haploid eggs). If these latter eggs are not fertilized, they develop parthenogenetically into males.
The habitat of rotifers may include lake bottoms, as well as flowing water environments such as rivers or streams. Rotifers also commonly inhabit the limnoterrestrial habitat such as the film of water on lichens, liverworts, and mosses growing on tree trunks and rocks. They can be found in rain gutters and puddles, in soil or leaf litter, on mushrooms growing near dead trees, in tanks of sewage treatment plants, and even on freshwater crustaceans and aquatic insect larvae. In addition to being found primarily in freshwater habitats, some genera inhabit saline habitats, and about 100 species live in purely marine environments. In addition, rotifers can be found on pitcher plants and tree hole communities termed phytotelmata as well as in artificial habitats such as bird baths, old tires, and discarded cups and containers. Rotifers can even be found in the interstitial water of soils and sediments and probably play an important role in nutrient cycling in soils.
The diet of rotifers consists of matter small enough to fit through their tiny mouths during filter feeding. They are primarily omnivorous primary consumers of small microorganisms and suspended organic matter, but some species have been known to be cannibalistic. Specific food items consist of dead or decomposing organic materials, as well as unicellular algae and other phytoplankton. Rotifers are, in turn, prey to carnivorous secondary consumers, including shrimp and crabs. A few rotifers are parasitic on a variety of organisms, including algae, sponges, freshwater oligochaetes, snail eggs, crustaceans, fishes, and even other rotifers. Genera such as Brachionus, Limnias, Pleurotrocha, Proales, and Ptygura make temporary attachments to either invertebrates or vertebrates. For example, the carapace of the planktonic crustacean Daphnia is often colonized by individuals of Brachionus rubens.
In Arkansas, although there is no summation of the species of rotifers occurring in the state, several surveys have been done in various localities. In one study, a zooplankton collection station at Pendleton Ferry (Desha County) on the Arkansas River exhibited a high “trophic index” of rotifers. The Rotatoria were found to be the most important zooplankters in terms of numbers and diversity, and the species most often observed were Brachionus angularis, B. calyciflorus, B. urceolaris, Conochilus unicornis, Hexarthra mira, Kellicottia bostoniensis, Keratella cochlearis, K. earlinae, K. valga, Pedipartia sp., Polyarthra vulgaris, Synchaeta oblonga, and S. pectinata. In another study, twenty-one genera of rotifers were noted in the upper Illinois Bayou arm of Lake Dardanelle. A different study in Lake Dardanelle reported twelve rotifer genera as follows: Asplanchna, Brachionus, Kellicottia, Keratella, Notholca, Monostyla, Enteroplea, Polyarthra, Trichocera, Lacinularia, Hexarthra, and Filinia. In another part of the state, rotifers reported from DeGray Reservoir were Asplanchna priodonta, C. unicornus, K. bostoniensis, K. cochlearis, and Synchaeta stylata. In Gillham Lake of the Little River system and Lake Greeson of the Little Missouri River system, fourteen and twelve taxa of rotifers were reported, respectively.
If one is interested in studying rotifers, collection is not difficult. They can be collected by pulling a fine-mesh (25 to 50 µm) net through any body of fresh water. Ponds and lakes are particularly good places to collect rotifers. Another simple method is to take a one-gallon glass jar and submerge it in a weedy area of a lake or pond and to place inside a few aquatic plants before retrieval. After retrieval, place the jar near a subdued light source (north-facing window). Removal of the rotifers that swim to the surface on the lighted side may be accomplished using a transfer pipette.
For additional information:
Edmondson, W. T. “Rotifera.” In Freshwater Biology, 2nd ed., edited by W. T. Edmondson. New York: John Wiley and Sons, Inc., 1959.
Hynes, H. B. N. The Ecology of Running Waters. Ontario: University of Toronto Press, 1970.
Kochsiek, K. A., J. L. Wilhm, and R. Horrison. “Species Diversity of Net Zooplankton and Physio-Chemical Conditions in Keystone Reservoir, Oklahoma.” Ecology 52 (1971): 1119‒1125.
Palko, Tom N. Build-Up of Mineral Content in Lake Dardanelle and Effect on Zooplankton. Arkansas Water Resources Research Center Publication No. 24, 1974.
———. “A Preliminary Study of Zooplankton over a Six-Month Period on Lake Dardanelle.” Proceedings of the Arkansas Academy of Science 24 (1970): 55‒61. Online at: https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2991&context=jaas (accessed February 25, 2021).
Rickett, John D., and Robert L. Watson. “Zooplankton Community Abundance and Diversity in Dardanelle Reservoir, Arkansas, 1981–1990.” Proceedings of the Arkansas Academy of Science 46 (1992): 57‒60. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2073&context=jaas (accessed February 25, 2021).
———. “Zooplankton Community Structure in Dardanelle Reservoir, Arkansas, 1975–1982.” Proceedings of the Arkansas Academy of Science 37 (1983): 65‒69. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2530&context=jaas (accessed February 25, 2021).
Roseberg, Ralph B., and Mark Karnes. “Influence of DeGray Reservoir on Zooplankton Populations in the Caddo and Ouachita Rivers.” Proceedings of the Arkansas Academy of Science 38 (1984): 96‒97. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2510&context=jaas (accessed February 25, 2021).
Segers, Hendrik. “Annotated Checklist of the Rotifers (Phylum Rotifera) with Notes on Nomenclature, Taxonomy, and Distribution.” Zootaxa 1564 (2007): 1‒104.
———. “Global Diversity of Rotifers (Rotifera) in Freshwater.” Hydrobiologia 595 (2008): 49‒59.
Short, Edgar D. “Zooplankton Studies.” In A Preliminary Study of the Water Quality of the Illinois River in Arkansas, edited by Paul Kittle, Edgar Short, and Ramona Rice; a final report to the Illinois River property owners of Siloam Springs, Arkansas, Inc., 1974.
Short, Edgar D., and Eugene H. Schmitz. An Evaluation of the Effects of Dredging Within the Arkansas River Navigation System. Volume III, Effects Upon the Zooplankton Associations. Final Report to the U.S. Corp of Engineers Contract No. DACW03-74-C-0146. Fayetteville: Arkansas Water Resources Center, 1976.
Smith, Stephen B., and Thomas E. Moen. “Zooplankton Population Structure in Three Reservoirs Near the Ouachita Mountain-Gulf Coastal Plain Interface.” Proceedings of the Arkansas Academy of Science 37 (1983): 99‒100. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2552&context=jaas (accessed February 25, 2021).
Thorp, J. H., and S. Mantovani. “Zooplankton of Turbid and Hydrologically Dynamic Prairie Rivers.” Freshwater Biology 50 (2005): 1471‒1491.
Wallace, Robert L. “Colonality in the Phylum Rotifera.” Hydrobiologia 147 (1987): 141‒155.
———. “Ecology of the Sessile Rotifers.” Hydrobiologia 73 (1980): 181‒193.
Wallace, Robert L., and H. A. Smith. Rotifera. In Encyclopedia of Inland Waters, edited by G. E. Likens. Oxford: Elsevier, 2009.
Wallace, Robert L., and T. W. Snell. “Rotifera.” In Ecology and Classification of North American Freshwater Invertebrates, edited by Jim Thorp and Alan Covich. New York, Academic Press, 1991.
Walsh, E. J. L. May, and Robert L. Wallace. “A Metadata Approach to Documenting Sex in Phylum Rotifera: Diapausing Embryos, Males, and Hatchlings from Sediments.” Hydrobiologia 796 (2017): 265–276.
Williams, L. G. “Dominant Planktonic Rotifers of Major Waterways of the United States.” Limnology and Oceanography 11 (1966): 83‒91.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College





Comments
No comments on this entry yet.