calsfoundation@cals.org
Invasive Animals
aka: Alien Animals
An “invasive species” is defined as a species that is non-native (or alien) to the ecosystem under consideration and whose introduction causes or is likely to cause economic or environmental problems or harm to human health. It typically matures and reproduces quickly and increases its geographic range rapidly, establishing populations and persisting over large areas. There are several reasons for this spread, including favorable environmental conditions and lack of natural predators, competitors, and diseases that normally regulate their populations, allowing invasive species to thrive. Invasive biota not only includes a variety of plants but also incorporates a wide variety of invertebrates and higher taxa from their native sites. As invasive species extend and dominate ecosystems, they invariably reduce native biodiversity by threatening the survival of most biota. In fact, invasive species are a significant threat to almost half of the native U.S. species listed as federally endangered.
In addition to harming ecosystems, invasive species are also economically costly. For federal, state, and local governments, it is very expensive to prevent, monitor, and control their spread, not to mention the damage they inflict upon crops, fisheries, wildlife, forests, and other resources. It has been estimated that invasive species cost the federal government about $137 billion annually.
Invasive species are often referred to as alien, exotic, or non-indigenous species. These labels are actually misnomers, in that they refer only to the non-native part of the definition herein. On the other hand, many exotic or alien species may not actually cause detriment to the economy, the environment, or even human health. In fact, the vast majority of “introduced” species do not endure, and only about fifteen percent of those that make progress to become “invasive” are potentially destructive.
In Arkansas, many invasive insects have been brought into the state accidentally in cargo, produce, and/or shipping containers, and finding invasive insects at ports of entry is difficult. A few invasive insects have been brought into the United States for research purposes but have escaped into the wild. Some of these insects have become invasive pests that devastate natural environments and destroy crops.
The following is a sample of some invasive invertebrates and other animals that occur in Arkansas.
Phylum Platyhelminthes: Class Turbellaria
The hammerhead or land flatworm (Bipalium kewense) is a hermaphroditic terrestrial planarian that originated from Southeast Asia; it possesses a spade-like head, hence its common name. They were apparently brought into the United States with horticultural plants and have been commonly found in greenhouses since the beginning of the twentieth century. Specimens are typically lightly colored (yellowish brown) and possess one to five dark dorsal stripes and a dark collar. They are a known predator of earthworms but may also feed on other organisms. Diurnally, B. kewense spends its time typically out of the direct sun under dripping garden faucets, leaf litter, rocks and logs, and shrubs. After heavy precipitation, they may be found moving about on the soil, driveways, patios, or sidewalks. They feed and move about in search of prey at night. In Arkansas, B. kewense has been reported from several counties, including Ashley, Chicot, Clark, Columbia, Dallas, Faulkner, Jefferson, Ouachita, Polk, Pope, and Pulaski.
Phylum Mollusca, Class Bivalvia
The Asiatic clam (Corbicula fluminea) is native to southeastern China, Korea, southeastern Russia, and the Ussuri Basin. The initial collection of C. fluminea in the United States was in 1938 along the banks of the Columbia River near Knappton, Washington. It was thought to have entered from Eurasia as a food item used by Chinese immigrants; however, it might have been imported along with the Giant Pacific oyster, Magallana gigas (also from Asia). By 2020, C. fluminea was found in all U.S. states (except Alaska) and the District of Columbia. It is found nearly statewide in Arkansas in various watersheds, including both lentic and lotic habitats. Current methods of introduction include bait bucket introductions, accidental introductions associated with imported aquaculture species, and intentional introductions by people who purchase them as a food item in markets. The only other noteworthy dispersal agent is thought to be passive movement via water currents. However, little is known about how the Asian clam possibly disrupts native ecosystems. For example, in the relatively unaltered Buffalo River in Arkansas, C. fluminea are abundant only in certain patches and apparently have been reported to pose no negative effect on the native unionid mussels in the watershed. However, it has been documented that indigenous fish and crayfish have added this clam to their diets. For example, dense populations of Asiatic clams exist in Norfork Lake and provide an excellent food supply for redear sunfish, Lepomis microlophus. While this may seem to be a positive solution to reducing their populations, C. fluminea could potentially be an intermediate host for exotic parasites that could then infect native species or cause diseases from human enteric parasites such as Cryptosporidium parvum and Giardia lamblia. In terms of economic importance, C. fluminea is mostly known as a biofouler of many electrical and nuclear power plants across the United States. As water is drawn from rivers, streams, and reservoirs for cooling purposes through intake pipes, so are thousands of Corbicula larvae. Once inside the power plant, this mussel can clog condenser tubes, raw service water pipes, and even fire-fighting equipment; the cost of removing them is estimated at about a billion dollars each year.
The zebra mussel (Dreissena polymorpha) is a native of Eurasia (specifically the Caspian Sea, Russia), and it was first observed in North America in 1988 in the Great Lakes. Since then, D. polymorpha has swept across various states located along the Mississippi Waterway. Zebra mussels prefer slow-moving water, although they can be found in rivers and streams, but their populations are higher in lakes, as high turbidity curtails zebra mussel populations. This mussel has the potential to have a devastating effect on the state’s natural resources, with estimated total cost in the United States of the zebra mussel infestation in the billions. Zebra mussels harm native mussels by competing for food and attaching to their shells and can also foul swimming areas with their sharp shells. They wreak havoc on boaters by attaching to boat trailers, damaging their boat hulls, and reducing the performance of boat motors by clogging water intakes. Unfortunately, this allows for easy invasion of multiple lakes if boaters are neglectful of checking and cleaning their boats.
In Arkansas, zebra mussels became established in the state around 1992. By 2018, biologists with the Arkansas Game and Fish Commission’s Fisheries Division confirmed the presence of zebra mussels in the portion of the White River just downstream from Bull Shoals Dam. Zebra mussels have apparently been present in Bull Shoals Lake at least since the early 2010s, and researchers observed a large population increase between 2014 and 2015. The number of zebra mussels appears highest directly below the dam, but biologists have found them up to 13.9 km (8 mi.) below the dam. District biologists are unsure how the mussels will impact the trout population in the White River, but they have not yet been able to document negative effects on fish populations in the lake from this invasive mussel.
Phylum Crustacea, Class Malacostraca
The red swamp crayfish (Procambarus clarkii) is a species of freshwater crayfish that is native to northern Mexico and the southern and southeastern United States, but was also introduced elsewhere (both in North America and other continents), where it is often an invasive pest. It is a tertiary burrower and occupies lentic and lotic habitats but can also be found in burrows on the Mississippi Alluvial Plain in eastern Arkansas. The red swamp crayfish is commonly raised by commercial crayfish producers in Arkansas and other states (especially Louisiana) for human consumption and has become a serious introduced agricultural pest. The burrowing habits of P. clarkii can lead to damage to water courses and to crops (particularly rice), and its feeding activities can disrupt native ecosystems. It has also been reported that this species can out-compete native crayfish species.
Phylum Arthropoda: Class Arachnida
The brown widow spider (Latrodectus geometricus) originated in South Africa and was imported into the United States via Florida; it is now found almost globally in subtropical regions. In the mid-to-late 1990s, there was an outbreak of brown widow spiders. To date, this spider has spread throughout Florida, with reported sightings in Alabama, Arkansas, southern California, Colorado, Georgia, Mississippi, Texas, and South Carolina. The spider is believed to have become established in these states by attaching to the underside of vehicles and being transported to distant locations. The brown widow spider prefers tropical climates and is commonly found in the southeastern United States, where it was introduced. Like its cousin, the black widow (L. mactans), the brown widow can be found on many manufactured structures in dark corners or crevices around houses, barns, fences, or anywhere a web will fit near the ground. This spider can vary in color from white to dark brown to almost black; however, most are light or medium brown. The ventral side of the abdomen contains an orange or yellow hourglass-shaped marking. It has brown shaded bands on leg segments, white spots on the abdomen, and marginal white stripes. The white abdominal stripes can distinguish this species from L. mactans, but the white stripes are often difficult to see on darker-colored brown widow spiders. The female is larger than the male. Currently, L. geometricus does not pose the same medical concerns as L. mactans. Bites from the brown widow do not cause the same symptoms as bites from the black widow. Brown widow spider venom is two-fold more potent as venom from the black widow; however, it is thought that the brown widow does not inject the same amount of neurotoxin. This results in a decreased severity of symptoms in the form of cramping or nausea among those bitten.
Varroa mite (Varroa destructor) is an ectoparasite of honeybees (Apis spp.). Originally, V. destructor was an external parasite of the Asian honeybee (Apis cerana) in mainland Asia and Japan but eventually was able to infest the European honeybee (A. melifera) as a host in 1963 in Singapore. An individual V. destructor was discovered in Maryland in 1979, and by 1987, specimens had appeared in both Florida and Wisconsin. Since then, it spread rapidly throughout the continental United States in both wild and managed honeybee colonies, including those overseas in the Hawaiian Islands. According to the U.S. Department of Agriculture, Arkansas lost 5,000 bee colonies in 2016 but added new colonies equal to the number lost.
Heavy infestations of these mites can cause newly emerging bees to have malformed wings, legs, and bodies. When worker bees leave hives or die off, this severely weakens the colony because no bees are able to maintain the larvae. Weak colonies are then susceptible to having their honey stores robbed by stronger hives, and, as a result, the colony eventually dies. With bees being an ecologically and economically important insect, the control of this mite is a must to ensure the continuing success of the apiary market. In the winter, mites solely infest adult bees in the hive; without hosts, adult mites can survive only a few days. For beekeepers, it is important to survey their colonies for the presence of the mite constantly. To prevent mites from spreading to other hives, any new bee-farming colonies that are found to have the mites should be immediately quarantined.
Class Insecta: Order Coleoptera
The emerald ash borer (Agrilus planipennis) is a bright, metallic-looking, emerald green invasive beetle from Asia. It was accidentally introduced into southeastern Michigan sometime in the 1990s in wood-packing material imported from eastern Asia and has been discovered in at least eighteen Arkansas counties. Due to the expanded range of A. planipennis infestation sites within the state, on March 27, 2018, the Arkansas Department of Agriculture’s State Plant Board approved a statewide quarantine for ash items.
This pest costs municipalities, nursery operators, property owners, and forest product industries tens of millions of dollars. The adults feed on the foliage of ash (Fraxinus spp.) but seem to cause little damage. However, larval A. planipennis feed on the phloem and sapwood of ash trees and disrupt the tree’s ability to transport water and nutrients. After only a year of infestation, S-shaped exit holes on branches and the trunk cause bark to split vertically above larval feeding galleries. Thus, additional stress likely contributes to vulnerability and rapid decline of the tree. This pest kills essentially one hundred percent of the ash trees it infests. Ash, a valuable hardwood in Arkansas forests, will likely be all but eliminated from the state if this insect cannot be controlled. Unfortunately, humans unknowingly contribute to the artificial spread of the emerald ash borer. Transporting common ash tree products, such as branches, firewood, green lumber, nursery stock, logs, and chips, has been a primary means of advancing the beetle’s spread.
The Japanese beetle (Popillia japonica) was introduced on nursery stock into Riverton, New Jersey, in 1916. It is thought that the grubs contaminated shipments of iris bulbs before 1912, when the United States started inspecting imports. In the next sixty years, it had spread throughout twenty-two states east of the Mississippi River. In Arkansas, P. japonica arrived by 1997 in nursery stock and is now present in at least ten counties in the state. It is an oval-shaped, metallic-green beetle with bronze wings. This beetle feeds on the foliage and fruit of hundreds of plant species, particularly damaging roses, orchards, and turfgrass. These beetles do not discriminate when feeding and cause severe damage to hundreds of plants. In fact, fruits such as grapes can be entirely consumed and their vines destroyed.
Order Hymenoptera
The red imported fire ant (Solenopsis invicta), originally from Brazil, was accidentally introduced into Alabama in the 1930s. The first documented infestation of these ants in Arkansas was in El Dorado (Union County) in 1958, and it now occurs in at least thirty counties of southern Arkansas. This ant has spread to infest more than 260 million acres of land in nine southeastern states, including all or portions of Alabama, Arkansas, Florida, Georgia, Mississippi, Oklahoma, South Carolina, Tennessee, and Texas. This ant is a prolific breeder as well as an aggressive, multiple stinger with venom that produces a small, painful pustule (white bump) on the victim within eight to twenty-four hours, and a potentially dangerous (but rare) hypersensitive reaction in humans. This ant may also damage fruit trees and their berries and young crops and is also known to damage electrical boxes; its mounds are a hazard to farm equipment. Their mounds are not only unsightly, but they can ruin lawns and, more ecologically damaging, they can displace and outcompete native ants in their habitat. This has led to a reduction in population numbers of all indigenous ants as well as other insects, amphibians, birds, and reptiles (most notably horned lizards, Phrynosoma spp.).
Africanized honey bees (Apis mellifera scutellata) are hybrids of the European honeybee (Apis mellifera) and the African honeybee (A. m. scutellata). Essentially, any other honeybee that it can breed with will produce viable offspring. The growth of the honey industry in Brazil in the 1950s drove the hybridization of A. m. scutellata by entomologists because the European honeybee was not able to survive for long enough to support the need for the product. In 1957, there was an accidental release of more than two dozen queens. These released bees were able to hybridize with the naturalized European bees. They migrated north from Brazil to Central America, then on to Mexico, and by 1990, were identified along the southern Texas border at Hidalgo. These bees have become prominent in the southwestern and southeastern United States and were first noted in Arkansas in 2005.
They are very similar morphologically to A. mellifera; however, their behavior truly distinguishes them from their docile European cousins. Africanized bees are extremely aggressive and are known to swarm and chase anything that disturbs the hive, making them more competitive for the same niche. Also, since the Africanized bee can hybridize with the European bees, it allows for competition. This makes the Africanized bees a threat to the honey industry. Since their introduction, relatively few deaths have been attributed to Africanized bees. A small number of people may have severe hypersensitive reactions to their stings, requiring immediate medical attention. However, their presence in Arkansas does not suggest that anyone should end beekeeping as a hobby or as an industry. Their occurrence will only require slightly more caution and safety on the part of beekeepers and the public alike.
Order Lepidoptera
The European gypsy moth (Lymantria dispar dispar) was first introduced in 1868 via Europe near Boston, Massachusetts, in an effort to breed a better silkworm. A decade after this introduction, the first outbreaks began, and in 1890, the state and federal governments began their attempts to eradicate the moth. In Arkansas in the summer of 2012, occasional confirmed reports of gypsy moths were documented (particularly in the Ozarks). It is one of North America’s most devastating forest pests. The gypsy moth is known to feed on the foliage of many taxa of plants in North America, but its most common hosts are oaks (Quercus spp.) and aspen (Populus spp.). In many forests, less than twenty percent of the trees in a forest will die, but occasionally tree mortality may be very heavy. A major concern is the potential loss of economically critical and ecologically dominant oak species.
Order Diptera
The Asian tiger mosquito (Aedes albopictus) was first documented in the United States (Texas) in 1985. A year later, it was found in Florida (at a tire dump site) near Jacksonville. Since then, the species has spread rapidly throughout the southeastern quadrant of the United States. This mosquito is easily recognized by the shiny black and distinct silver-white scales on the body. Male mosquitoes feed on plant juices and do not pierce human skin; however, females take blood meals to nourish eggs. Unlike many other Arkansas mosquitoes, the Asian tiger mosquito feeds during the day (peaks in the early morning and late afternoon), not at night. The Asian tiger mosquito is an appropriate vector for blood-borne diseases, including Chikungunya, Zika, and West Nile viruses, and dengue fever. While this mosquito can transfer diseases to human hosts, it can also harbor diseases that affect livestock and pets.
Phylum Chordata, Class Mammalia
The nutria (Myocastor coypus) is a large, dark-colored, semiaquatic rodent from South America. Nutria were first introduced by fur ranchers into the marshes near New Orleans, Louisiana, in the early 1930s; however, these were all trapped and recovered. In 1938, twenty additional M. coypus were imported from Argentina and placed in a nutria ranch on Avery Island, Louisiana; however, they either escaped or were released when the business failed. By the middle 1940s, M. coypus were extremely common in the southern half of Arkansas and, by the late 1950s, had quickly spread to all parts of the state, especially the West Gulf Coastal Plain, the Mississippi Alluvial Plain, and up the Arkansas River Valley. Interestingly, the rate of M. coypus expansion into Arkansas has been extremely rapid. Over a period from the 1960s to the mid-1970s, the species spread over 390 km (242 mi.) northward. This represents a conservative average invasion rate of between 20 to 24 km/yr. (12 to 15 mi./yr.). In other locales, state and federal agencies and other individuals translocated nutria into Alabama, Georgia, Kentucky, Louisiana, Maryland, Mississippi, Oklahoma, and Texas, with the intention to control unwanted vegetation and enhance trapping opportunities, or to be sold as weed cutters to an ill-informed public throughout the southeast. Nutria live in marshy or swampy areas, streams, farm and minnow ponds, and oxbow lakes. In other parts of the United States, farm ponds and other freshwater impoundments, drainage canals with spoil banks, rivers and bayous, freshwater and brackish marshes, swamps, and combinations of various wetland types can provide suitable habitat to nutria. The estimated value of sugarcane and rice damaged by nutria each year has ranged from several thousand dollars to over a million. If losses of other resources are added to this amount, the estimated average loss would likely exceed $1 million annually.
The ancestors of the feral hog (Sus scrofa) are native to much of Eurasia, North Africa, and the Greater Sunda Islands. Purebred S. scrofa were introduced by wealthy landowners as big game animals and as a food source into the New World (New Hampshire) in the nineteenth century. The initial introductions took place in fenced enclosures, but several individuals escaped, with the escapees sometimes intermixing with already established feral pig populations. More recently, feral hog populations have been reported in at least 44 states, most are likely wild boar × feral hog hybrids. Pure wild boar populations may still be present, but are extremely localized.
These non-native feral hogs or wild swine compete directly with wildlife for food resources, destroy habitat by rooting and wallowing, and will eat ground-nesting birds, their eggs, deer fawns, and young domestic livestock. Although feral hogs have been in Arkansas for centuries, during the twenty-first century, S. scrofa has become a major invasive pest in the state’s forests and agricultural cropland. They cost Arkansas’s producers millions of dollars each year by damaging forests and crops and polluting streams and ponds. In addition, feral hogs can serve as hosts to many different diseases and parasites, including brucellosis, trichinellosis, and swine herpes virus. The Arkansas Game and Fish Commission concedes that large-scale trapping is the most efficient and economical means currently available to reduce feral hog populations.
For additional information:
Abramson, C. I., I. S Aquino, G. A. Azeredo, and J. M. Price. “Some Preliminary Studies on the Ability of Africanized Honey Bees (Apis mellifera L.) to Tolerate Cold Temperatures When Placed Inside a Refrigerator.” Psychological Reports 81 (1997): 707–718.
Baerg, W. J. “The Black Widow and Five Other Venomous Spiders in the United States.” Agricultural Experiment Station, University of Arkansas. Fayetteville, Arkansas, Bulletin 608 (1959): 1–43.
Bailey, Joe W., and Gary A. Heidt. “Range and Status of the Nutria, Myocaster coypus, in Arkansas.” Proceedings of the Arkansas Academy of Science 32 (1978): 25‒27. Online at https://scholarworks.uark.edu/jaas/vol32/iss1/9/ (accessed September 22, 2021).
Banks, W. A., C. T. Adams, C. S. Lofgren, and D. P. Wojcik. “Imported Fire Ant Infestation of Soybean Fields in the Southern United States.” Florida Entomologist 73 (1990): 503–504.
Brown, K. S., J. S. Necaise, and J. Goddard. “Additions to the Known U.S. Distribution of Latrodectus geometricus (Araneae: Theridiidae).” Journal of Medical Entomology 45 (2008): 959–962.
Carter, J., and B. P. Leonard. “A Review of the Literature on the Worldwide Distribution, Spread of, and Efforts to Eradicate the Coypu (Myocastor coypus).” Wildlife Society Bulletin 30 (2002): 162–175.
Cohen, P. R. “Imported Fire Ant Stings: Clinical Manifestations and Treatment.” Pediatric Deteratology 9 (1992): 44–48.
Counts, C. L., III. “The Zoogeography and History of the Invasion of the United States by Corbicula fluminea (Bivalvia: Corbiculidae).” American Malacological Bulletin, Special Edition 2 (1986): 7–39.
Hammons, D. L., S. K. Kurtural, M. C. Newman, and D. A. Potter. “Invasive Japanese Beetles Facilitate Aggregation and Injury by a Native Scarab Pest of Ripening Fruits.” Proceedings of the National Academy of Sciences 106 (2009): 3686–3691.
Jamieson, David H., and Larry A. Olson. “Recent Establishment of the Asian Tiger Mosquito (Aedes albopictus) in Independence County, Arkansas.” Proceedings of the Arkansas Academy of Science 49 (1995): 80–81. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1889&context=jaas (accessed September 22, 2021).
Jamieson, David H., Larry A. Olson, and J. D. Wilhide. “A Larval Mosquito Survey in Northeastern Arkansas Including a New Record for Aedes albopictus.” Journal of the American Mosquito Control Association 10 (1994): 236–239.
Johnson, D. T. History and Management of Japanese Beetle in Arkansas and Oklahoma. In: Proceedings of the Oklahoma and Arkansas Horticulture Industries Show, Volume 23, 2004.
McAllister, Chris T., Henry W. Robison, and Renn Tumlison. “Additional County Records of Invertebrates from Arkansas.” Journal of the Arkansas Academy of Science 72 (2018): 193–197. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=3317&context=jaas (accessed September 22, 2021).
Moore, C. G., and C. J. Mitchell. “Aedes albopictus in the United States: Ten-Year Presence and Public Health Implications.” Emerging Infectious Diseases 3 (1997): 329–334.
Petty, B. M., D. T. Johnson, and D. C. Steinkraus. “Survey of Pathogens and Parasitoids of Popillia japonica (Coleoptera: Scarabaeidae) in Northwest Arkansas.” Journal of Invertebrate Pathology 111 (2012): 56–59.
Pfitzner, Shelley, David H. Jamieson, and Larry A. Olson. “The Colonization of an Ozark Mountain City by the Asian Tiger Mosquito (Aedes albopictus).” Journal of the Arkansas Academy of Science 52 (1998): 13. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1793&context=jaas (accessed September 22, 2021).
Robison, Henry W., Keith A. Crandall, and Chris T. McAllister. “An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas.” Journal of the Arkansas Academy of Science 71 (2017): 17‒34. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2160&context=jaas (accessed September 22, 2021).
Rosenkranz, Peter, Pia Aumeier, and Bettina Ziegelmann. “Biology and Control of Varroa destructor.” Journal of Invertebrate Pathology 103 (2010): S96–S119.
Sampayo, Rafael R. “Pharmacological Action of the Venom of Latrodectus mactans and Other Latrodectus Spiders.” The Journal of Pharmacology and Experimental Therapeutics 80 (1944): 309–322.
Savage, H. M., G. C. Smith, J. C. Mitchell, R. G. McLean, and M. V. Meish. “Vector competence of Aedes albopictus from Pine Bluff, Arkansas for a St. Louis Encephalitis Virus Strain Isolated During the 1991 Epidemic.” Journal of the American Mosquito Control Association 10 (1994): 501–506.
Sealander, John A., and Gary L. Heidt. Arkansas Mammals: Their Natural History, Classification, and Distribution. Fayetteville, Arkansas: University of Arkansas Press, 1990.
Sousa, R., C. Antunes, and L. Guilhermino. “Ecology of the Invasive Asian Clam Corbicula fluminea (Müller, 1774) in Aquatic Ecosystems: An Overview.” International Journal of Limnology 44 (2008): 85–94.
Spivak, Marla, and Gary S. Reuter. “Varroa destructor Infestation in Untreated Honey Bee (Hymenoptera: Apidae) Colonies Selected for Hygienic Behavior.” Journal of Economic Entomology 94 (2001): 326–331.
Sprenger, D., and T. Wuithiranyagool. “The Discovery and Distribution of Aedes albopictus in Harris County, Texas.” Journal of the American Mosquito Control Association 2 (1986): 217–219.
Villa, J. D., T. E. Rinderer, and J. A. Stelzer. “Answers to the Puzzling Distribution of Africanized Bees in the United States or “Why are Those Bees Not Moving East of Texas?” American Bee Journal 142 (2002): 480–483.
Wilson, J., and David H. Jamieson. “Presence of the Asian Tiger Mosquito (Aedes albopictus) in Northwest Arkansas.” Journal of the Arkansas Academy of Science 64 (2010): 151–152. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1394&context=jaas (accessed September 22, 2021).
Chris T. McAllister
Eastern Oklahoma State College
 Asian Longhorned Tick
Asian Longhorned Tick Carps
Carps Dipterans
Dipterans Exotic Fish
Exotic Fish Northern Snakehead
Northern Snakehead Science and Technology
Science and Technology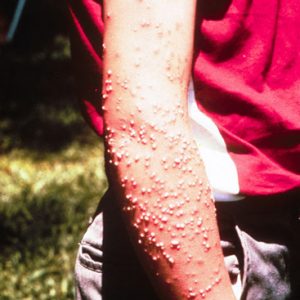 Fire Ant Stings
Fire Ant Stings  Fire Ants
Fire Ants  Invasive Arthropods
Invasive Arthropods  Invasive Invertebrates
Invasive Invertebrates  Invasive Mammals
Invasive Mammals  Japanese Beetle
Japanese Beetle  Northern Snakehead
Northern Snakehead 



Comments
No comments on this entry yet.