calsfoundation@cals.org
Grotto Salamander
aka: Eurycea spelaea
aka: Ghost Lizard
aka: Ozark Blind Salamander
The grotto salamander (Eurycea spelaea) is a species of lungless salamander in the Phylum Chordata, Subphylum Vertebrata, Class Amphibia, Order Caudata, and Family Plethodontidae. It was originally described as Typhlotriton spelaeus but is now considered a member of the genus Eurycea. It is endemic to wet caves, sinkholes, and karst regions beneath the Springfield and Salem Plateaus of the Ozark Mountains of 120 individual sites in northern Arkansas, 124 sites in southwestern Missouri, forty-three sites in northeastern Oklahoma, and one county (Cherokee) in extreme southeastern Kansas. Its natural habitats are freshwater springs, inland karsts, and caves. It is not listed by the federal government as endangered or threatened, but it is vulnerable to changes in groundwater quality and a reduction in bat population.
In Arkansas, E. spelaea has been reported from sites in sixteen counties: Baxter, Benton, Boone, Carroll, Fulton, Independence, Izard, Lawrence, Madison, Marion, Newton, Randolph, Searcy, Sharp, Stone, and Washington. Some specific Arkansas caves and/or springs with records of E. spelaea include the following (county in parentheses): Irish Hill (Baxter); Monte Ne (Benton); Marble (Boone); Burnet and Eureka Springs (Carroll); Mammoth Spring and Richardson (Fulton); Allen, Bell, Cushman, Dodd, Fair Spring, Forshee, Hankin’s, and Scout (Independence); Bergren, Clay, Greasy Bottom, and Needles (Izard); York Spring (Lawrence); Arrowbluff (Marion); Haddock Spring and John Eddings (Newton); Hurricane (Searcy); Bald Scrappy, Biology, Blanchard Springs Caverns, Center Nos. 1 and 2, Eckel, Gunner, Hell Creek, Spring, Roasting Ear, Ranch, Rowland, Slick Rock Hollow, and Wind Tunnel (Stone); and Farmington and Savoy (Washington).
The grotto salamander was initially described in 1892 from Rock House Cave (Barry County, Missouri) by Norwegian-born American herpetologist, ornithologist, and zoologist Leonhard Hess Stejneger (1851‒1943). The grotto salamander persisted for a long while as a distinct genus (Typhlotriton) based on morphological characteristics. There were also two other described species of the genus as follows: T. nereus described in 1944 from the type locality York Spring at Imboden (Lawrence County) and T. braggi described with a type locality of Cushman Cave (Independence County) in 1968. Both were described using invalid morphological characteristics and synonymized with E. spelaea. However, using molecular techniques, E. spelaea (sensu lato) was found to be composed of three different geographic clades across its distributional range. Therefore, it has been proposed that the name Eurycea braggi be resurrected for the Southern Clade and Eurycea nerea be resurrected for the Northern Clade, while the Western Clade should preserve the name of Eurycea spelaea. This entry will cover all three species under the E. spelaea species umbrella.
Unlike many cave-dwelling salamanders, the grotto salamander is the only known troglobitic caudate that retains a biphasic life cycle with aquatic larvae and fully transformed, sexually mature, terrestrial adults. Larval grotto salamanders (10 to 30 mm [0.4 to 1.2 in.]) possess functional eyes, external gills, and a distinctive high tail fin and pigments similar to other species of surface-dwelling larval Eurycea. They are found in both surface water (brooks or streams) and near cave entrances or just inside the twilight zone of subterranean habitats. After an extended larval period of between two to six years, depending on the locale, larvae travel farther inside caves and underground aquifers and metamorphose to adults. During this developmental period, they lose their gills, their eyes degenerate, and their eyelids fuse shut or partially so, and their brownish or purplish gray pigments (sometimes with spots or streaking on the sides and tail) fade.
Adults normally range from 75 to 121 mm (3.0 to 4.8 in.) in total length (record, 135 mm [5.3 in.]) and are pinkish-white, sometimes with traces of orange on their tail, feet, and lower sides, and have sixteen to nineteen (usually seventeen) costal grooves. Mature males have a slightly swollen upper lip and a pair of cirri, papilla-like extensions from the upper lip. Males also have a raised area on the chin used in courtship called a mental gland. They are obligate cavernicoles that eventually migrate deep into caves and live out their lives in underground aquifers. Adults have rarely been documented outside of caves (unless washed out during flooding events), and reproduction is likely exclusive to hypogean habitats. Interestingly, these salamanders are more often found in caves that support significant numbers of bats.
Larval grotto salamanders feed on amphipods, isopods, fly larvae, snails, and other small aquatic invertebrates in spring-fed streams. Within the cave environment, adult grotto salamanders prefer waters between 5.5° and 16.5° C (42° to 62° F). Here, they function as top predators on cave-dwelling invertebrates and other small prey found near guano, including flies, mosquito larvae, millipedes, pseudoscorpions, and beetles. They have also been reported to feed on bat guano. Larval specimens can be preyed upon by crayfish, fish (especially sculpins), and birds such as herons and egrets, whereas adult E. spelaea serve as prey to larger vertebrates, such as mammals venturing into caverns such as raccoons, skunks, opossums, and wild canids and felids. After adults die, their carcasses serve as organic nutrients for cave biota.
This grotto salamander reproduces during the period of maximal food supply, which is usually in spring to early summer months. As with most salamanders, fertilization is internal and the eggs are likely attached to stones in or near cave waters. Similar to other plethodontids, attendance of eggs by females is likely, and clutch size was reported from a single female to be thirteen ova. Hatchlings range from 10 to 16 mm (0.4 to 0.6 in.) in snout to vent length and are eventually washed outside the cave. In captivity, adults have been reported to live for at least twelve years, but their lifespan in nature is unknown.
The grotto salamander is especially sensitive to water quality degradation due to environmental contamination, or from human disturbance and over-collection. In Arkansas, Missouri, and Oklahoma, it is listed as a species of greatest conservation need or conservation concern, while in Kansas, it is considered state endangered. According to NatureServe, E. spelaea is ranked in Arkansas and Oklahoma as S3 (vulnerable), in Kansas as S1 (critically imperiled), and in Missouri S2/S2 (imperiled to vulnerable). In addition, E. braggi and E. spelaea both have relatively small geographic distributions, which may increase conservation concern for these species with potential for future management and preservation.
In terms of parasites, specimens from Arkansas and Missouri have been reported to harbor multiple species of helminth parasites (protozoans, trematodes, tapeworms, nematodes, and acanthocephalans). A new species of tapeworm was described in 1949 from E. spelaea from Mayes County, Oklahoma. In addition, an extreme case of trematode parasitism was also reported in a grotto salamander from Missouri, and yellow grub (Clinostomum marginatum) and the gill monogenean, Sphryanura euryceae, were documented from E. spelaea from Oklahoma. A single larval E. spelaea from a cave in Carter County, Missouri, was reported to test positive for the amphibian chytrid fungus, Batrachochytrium dendrobatidis (Bd), which is of interest because there are few documented cases of Bd in larval salamanders and none previously in any grotto salamander; however, three adult E. spelaea from the same site were negative.
For additional information:
Altig, Ronald, and Patrick H. Ireland. “A Key to Salamander Larvae and Larviform Adults of the United States and Canada.” Herpetologica 40 (1984): 212‒218.
Arkansas Endangered, Threatened, Regulated, and Species of Greatest Conservation Need. Little Rock: Arkansas Game and Fish Commission, 2016.
Bishop, Sherman C. “A New Neotenic Plethodont Salamander, with Notes on Related Species.” Copeia 1944 (1944): 1‒5.
Bonett, Ronald M., Michael A. Steffen, Ana L. Trujano-Alvarez, Samuel D. Martin, Charles R. Bursey, and Chris T. McAllister. “Distribution, Abundance, and Genetic Diversity of Clinostomum spp. Metacercariae (Trematoda: Digenea) in a Modified Ozark Stream System.” Journal of Parasitology 97 (2011): 177‒184.
Brandon, Ronald A. “Correlation of Seasonal Abundance with Feeding and Reproductive Activity in the Grotto Salamander (Typhlotriton spelaeus).” American Midland Naturalist 86 (1971): 93‒100.
———. “A Reevaluation of the Status of the Salamander, Typhlotriton nereus Bishop.” Copeia 1966 (1966): 555–561.
———. “Typhlotriton, T. nereus, T. spelaeus.” Catalogue of American Amphibians and Reptiles 20 (1965): 1‒2.
———. “Typhlotriton, T. spelaeus.” Catalogue of American Amphibians and Reptiles 84 (1970): 1‒2.
Brandon, Ronald A., and Jeffery H. Black. “The Taxonomic Status of Typhlotriton braggi (Caudata, Plethodontidae).” Copeia 1970 (1970): 388‒391.
Briggler, Jeffery T., and J. W. Prather. “Seasonal Use and Selection of Caves by Plethodontid Salamanders in a Karst Area of Arkansas.” American Midland Naturalist 155 (2006): 136–148.
Dunivan, James D., C. Renn Tumlison, and V. Rick McDaniel. “Cave Fauna of Arkansas: Further Records.” Proceedings of the Arkansas Academy of Science 36 (1982): 87‒88.
Dyer, William G. “Parasitism as an Indicator of Food Sources in a Cave-Adapted Salamander Habitat.” Bulletin of the Southern California Academy of Sciences 74 (1975):72‒75.
Fenolio, Danté B., Chris T. McAllister, Matthew L. Niemiller, Daphne Soares, and Jim Cooley. “An Extreme Case of a Trematode Infection in a Larval Ozark Blind Salamander, Eurycea spelaea (Caudata: Plethodontidae) from Ozark Highlands of Missouri, USA.” Speleobiology News 5 (2013): 34‒37.
Fenolio, Danté B., Matthew L. Niemiller, Ronald M. Bonett, G. Graening, B. A. Collier, and Jim F. Stout. “Life History, Demography, and the Influence of Cave-Roosting Bats on a Population of the Grotto Salamander (Eurycea spelaea) from the Ozark Plateaus of Oklahoma (Caudata; Plethodontidae).” Herpetological Conservation and Biology 9 (2014): 394–405.
Fenolio, Danté B., and Stanley E. Trauth. “Typhlotriton (Eurycea) spelaeus, Grotto Salamander.” In Amphibian Declines: The Conservation Status of United States Species, edited by Michael J. Lannoo. Berkeley: University of California Press, 2005.
Graening, G. O., Danté B. Fenolio, and Michael E. Slay. Cave Life of Oklahoma and Arkansas: Exploration and Conservation of Subterranean Biodiversity. Norman: University of Oklahoma Press, 2011.
Ireland, Patrick H., and Ronald Altig. “Key to the Gilled Salamander Larvae and Larviform Adults of Arkansas, Kansas, Missouri, and Oklahoma.” Southwestern Naturalist 28 (1983): 271‒274.
McAllister, Chris T., Charles R. Bursey, Stanley E. Trauth, and Danté B. Fenolio. “Helminth Parasites of the Grotto Salamander, Eurycea spelaea (Caudata: Plethodontidae), from Northern Arkansas and Southern Missouri, U.S.A.” Comparative Parasitology 73 (2006): 291‒297.
McAllister, Chris T., Charles R. Bursey, Michael A. Steffen, Samuel D. Martin, Ana L. Trujano-Alvarez, and Ronald M. Bonett. “Sphryanura euryceae (Trematoda: Sphyranuridae) from the Grotto Salamander, Eurycea spelaea and Oklahoma Salamander, Eurycea tynerensis (Caudata: Plethodontidae), in Northeastern Oklahoma, U.S.A.” Comparative Parasitology 78 (2011):188‒192.
McDaniel, V. Rick, and James E. Gardner. “Cave Fauna of Arkansas: Vertebrate Taxa.” Proceedings of the Arkansas Academy of Science 31 (1977): 68‒71. Online at https://scholarworks.uark.edu/jaas/vol31/iss1/22/ (accessed September 22, 2021).
Miller, Timothy J. “Genetic Variation of the Grotto Salamander, Typhlotriton spelaeus in Missouri.” MS thesis, Springfield: Missouri State University, 1999.
Petranka, James W. Salamanders of the United States and Canada. Washington DC: Smithsonian Institution Press, 1998.
Phillips, John G., Danté B. Fenolio, Sarah L. Emel, and Ronald M. Bonett. “Hydrologic and Geologic History of the Ozark Plateau Drive Phylogenomic Patterns in a Cave-Obligate Salamander.” Journal of Biogeography 44 (2017): 1‒12.
Powell, Robert, Roger Conant, and Joseph T. Collins. Peterson Field Guide to Reptiles and Amphibians Eastern/Central North America. 4th ed. Boston: Houghton Mifflin Company, 2016.
Reeves, J. D. “A New Tapeworm of the Genus Bothriocephalus from Oklahoma Salamanders.” Journal of Parasitology 35 (1949): 600–604.
Rimer, Rhonda L., and Jeffrey T. Briggler. “Occurrence of the Amphibian Chytrid Fungus (Batrachochytrium dendrobatidis) in Ozark Caves, Missouri, USA.” Herpetological Review 41 (2010): 175‒177.
Smith, Charles C. “A New Typhlotriton from Arkansas (Amphibia: Caudata).” Wasmann Journal of Biology 26 (1968): 155–159.
———. “Notes on the Salamanders of Arkansas. 1. Life History of a Neotenic Stream-Dwelling Form.” Proceedings of the Arkansas Academy of Science 13 (1960): 66–74. Online at https://scholarworks.uark.edu/jaas/vol13/iss1/9/ (accessed September 22, 2021).
Trauth, Stanley E., Robert L. Cox Jr., Brian P. Butterfield, David A. Saugey, and Walter E. Meshaka. “Reproductive Phenophases and Clutch Characteristics of Selected Amphibians.” Proceedings of the Arkansas Academy of Science 44 (1990): 107‒113. Online at https://scholarworks.uark.edu/jaas/vol44/iss1/29/ (accessed September 22, 2021).
Trauth, Stanley E., Henry W. Robison, and Michael V. Plummer. The Amphibians and Reptiles of Arkansas. Fayetteville: University of Arkansas Press, 2004.
Chris T. McAllister
Eastern Oklahoma State College
 Dipterans
Dipterans Science and Technology
Science and Technology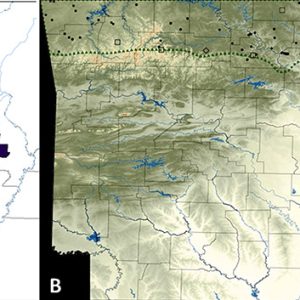 Grotto Salamander Distribution
Grotto Salamander Distribution  Grotto Salamanders
Grotto Salamanders 



Comments
No comments on this entry yet.