calsfoundation@cals.org
Nematodes
aka: Roundworms
The phylum Nematoda includes three classes (Anoplea, Chromodorea, and Rhabditida), sixteen to twenty orders, and about 27,000 described species (and possibly up to one million) of mostly dioecious, elongate, bilaterally symmetrical pseudocoelomate worms. They can be found in abundance in nearly every habitat on Earth, with a diverse array of species existing in both marine and terrestrial habitats. Most are free-living, with less than half considered to be parasitic. Nevertheless, many species threaten the health of plants and animals (including humans) on a global scale. Nematodes are variable in size from less than one millimeter to more than one meter in length. They have been in existence for an estimated one billion years, having evolved from simple animals some 400 million years before the “Cambrian explosion” of invertebrates.
Hookworms and pinworms are common examples that affect humans in Arkansas and around the world. Plant nematodes are also found in the state, with a total of 110 species found in various habitats. Plant nematodes damage crops in Arkansas such as rice, strawberries, and soybeans. Various entities in Arkansas perform research on nematodes, including the Arkansas Nematode Diagnostic Clinic in Hope (Hempstead County).
Carolus Linnaeus (1707–1778), in his Systema Naturae (10th edition), placed the roundworms in his class Vermes, but it was Karl Asmund Rudolphi (1771–1832), the father of helminthology (the study of parasitic worms), who made the greatest early advances in the recognition of various nematodes. He gave the name Nematoidea and produced a publication, Entozoorum Synopsis, with 350 species belonging to eleven genera in 1819. Important nematologists of the modern era include Nathan Augustus Cobb (1859–1932), the “father of U.S. nematology,” who pioneered agricultural nematology as a United States Department of Agriculture (USDA) scientist in the early 1900s. Roy C. Anderson (1926–2001) provided keys to the nematodes that infect vertebrates as well as a seminal text on the development, life cycles, transmission, and evolution of nematodes, and J. Ralph Lichtenfels (1939–) curated the systematic parasite (including nematode) collections of U.S. National Parasite Collection at the Agricultural Research Service in Beltsville, Maryland.
Nematode Morphology
The body wall of roundworms consists of an outer cuticle composed of the epicuticle (trilaminate), exocuticle (two zones; outer zone unstriated; inner zone striated), mesocuticle (obliquely striated; allows some movement), and endocuticle (unorganized fibers). The hypodermis secretes the cuticle and lies underneath a basal lamina, directly under the cuticle. The muscle wall is composed of spindle-shaped cells that run longitudinally. Cuticular ornamentation may include ridges that form distinct patterns in nematodes, termed the synlophe. The primitive condition is for a nematode to have six lips; however, in most parasitic nematodes, lips have fused, creating two or three lips, or no lips at all. An alae (or wing) is often present and is a compressed, lateral, and longitudinal cuticular thickening, and may include a longitudinal alae along the body, a cervical alae at the anterior end, or a caudal alae at the posterior end. Ballonets are found only in the gnathostomes and include four inflated areas surrounding the lips with small spines each connected to an internal cervical sac of unknown function. Head bulbs (bulbed regions) surrounding the lips are found in a few nematodes that do not have ballonets. In some nematodes, cordons (longitudinal, cord-like thickenings or ridges at the anterior end) include head shields, cephalic vesicles (inflated regions of the cuticle at the anterior end), collarettes (cuticular collar surrounding the head region posterior to the lips, prepuce-like), or bosses (irregular scutes, or bumps, at anterior end).
The basic structure of the digestive (alimentary) tract includes a complete gut, although some nematodes have anal atrophy. A stomodeum (buccal cavity plus esophagus) is lined by cuticle as well as the proctodeum (or rectum), which is also lined with cuticle. The nematode mouth is usually circular and may contain up to six lips. The buccal cavity is an area between the mouth and esophagus, often forming a ridged structure termed the buccal capsule (which may be reduced or absent in some species). Armament is common in parasitic and predatory forms, including cutting plates, ridges, rods, spears, stylets, and teeth. The esophagus (pharynx) is a muscular, pumping organ that pulls food into the gut and forces food into intestine; it may have one or more enlargements, termed bulbs. Digestive enzymes secreted by nematodes include amylases, cellulases, chitinases, proteases, and anti-coagulants (in some hookworms). In the Class Enoplea (=Adenophora, Aphasmidea), specifically the ordinal taxa Trichurida and Mermithida, the anterior portion is a thin-walled muscular tube, whereas the posterior portion is a thin tube surrounded by a column of singular, glandular cells termed stichocytes; the entire structure is termed a stichosome. The intestinal tract is a straight, non-muscular tube with microvilli that includes an esophageal valve, and there may be intestinal ceca at its junction. There are three distinct regions: (1) a ventricular region (which is mainly secretory), (2) a middle region (absorptive), and (3) a pre-rectal region (also absorptive). The nematode rectum is lined by cuticle. In females, a short, terminal, cuticle-lined rectum runs between the anus and intestine, while in males the rectum receives products of the reproductive system in its terminal portion, and, thus, is technically a cloaca.
The nervous system (using Ascaris as an example) includes a circumesophageal nerve ring (for nerve cells and four glial cells) with a pair of ventral ganglia, a pair of lateral ganglia, and a dorsal ganglion. A series of nerves travels posteriorly. The ventral nerve trunk is the largest nerve running posteriorly as chain of ganglia; the final ganglion (preanal ganglion) branches to form a posterior nerve ring. Sense organs include cephalic papillae with tactile receptors and sometimes labial papillae, caudal papillae, and usually cervical papillae (deirids) at the level of the nerve ring. Sensory pits called amphids are on either side of the head, and they tend to be reduced in parasitic species. There are up to twenty-three sensory nerve endings/amphid. In some groups (hookworms), the amphids also appear to secrete anti-coagulants. Structures called phasmids (in the Rhabditia only) are mostly similar in structure to amphids (chemosensory function).
The male reproductive system usually contains one testis (sometimes two). A muscular ejaculatory duct opens into the cloaca. A few nematodes possess cement glands to plug the vulva after copulation. In most nematodes, copulatory spicules (an important taxonomic character) arise from the cloacal wall; they function to hold the vulva open during copulation. A gubernaculum is present in many species, as it is a dorsal sclerotization of the cloacal wall that guides exertion of the spicules during copulation. In some stronglid nematodes, a telamon is present; it is an additional ventral sclerotization of the cloacal wall with a function similar to the gubernaculum. Sperm cells contain no flagella or acrosome, and they move by pseudopodia when mature. Copulatory bursae are flap-like expansions of the cuticle in nematodes of the order Strongylida or whole body as in the order Dioctophymatida. They are supported by thin extensions of the body, termed rays, in the Strongylida. They serve the male in grasping females during mating.
In the female reproductive system, most species have two ovaries (didelphic condition). Many roundworms have two uteri that converge from opposite directions. A germinal zone in the ovary produces oogonia, and these eventually mature into oocytes that move posteriorly through a growth zone attached to a rachis (a central, longitudinal, supporting structure in the ovary). They then move to spermatheca (a sperm storage area) for fertilization and finish shell formation and meiosis there. Nematodes have a muscular uterus that moves eggs containing embryos down to the ovijector (a highly muscular portion of the uterus). The vulva always opens anteriorly unless it opens into rectal area to form the cloaca.
Just prior to fertilization in most nematodes, excretory/secretory products of the male attract the female, whereas some species seek out the coiled posterior end of male tail (thigmotactic). The caudal papillae in the tail of males detect the vulval area, spicules probe for the vulva, and then insert spicules followed by sperm transfers. The development of the embryo begins with penetration of an oocyte by sperm, which initiates eggshell formation. Larvae (L) undergo four molts, with a new cuticle being produced under the old (the hypodermis detaches from the old sheath and produces a new one that is highly folded under the old one). Larval nematodes produce an ex-sheathing fluid (called leucine aminopeptidase), which is stimulated by carbon dioxide, allowing the old cuticle to loosen and rupture.
Free-Living and Parasitic Nematodes
Compared to their parasitic and terrestrial counterparts, marine free-living nematodes are dramatically understudied, as only about 4,000 species are described. Free-living nematodes in general are beneficial in the recycling of soil nutrients and decomposition of organic material. Interstitial nematodes pervade soil ecosystems and sediment in tremendous numbers. Soil is an excellent habitat for nematodes, and just 100 milliliters of soil may contain several thousand specimens. Nematode bacterivores and fungivores do not feed directly on soil organic matter but on the bacteria and fungi that decompose organic matter. The presence and feeding of these nematodes accelerate the decomposition process. Their feeding recycles minerals and other nutrients from bacteria, fungi, and other substrates and returns them to the soil where they are accessible to plant roots. The majority of free-living nematodes have not been studied in detail. Therefore, there is a high likelihood that most soil habitats contain undescribed species of free-living nematodes. However, specific identification of these groups is extremely difficult, and there are only a few nematode taxonomic specialists in the world who can formally describe new species of free-living nematodes. Therefore most non-specialists identify soil nematodes only to family or genus.
Plant parasitic nematodes are today recognized as major agricultural pathogens and cause crop losses throughout the world. Some estimates suggest they may cause $77 billion of damage worldwide each year. Plant nematodes feed on all parts of the plant, including flowers, leaves, roots, seeds, and stems. Nematodes feed from plants in a variety of ways, but all use a specialized spear called a stylet. The size and shape of the stylet is of taxonomic utility and also can be used to infer their mode of feeding. In one study in Arkansas, a total of 110 species of plant nematodes were found in various habitats. Reports of nematode damage to important crops in Arkansas include Aphelenchoides besseyi in rice and strawberries, Heterodera glycines and Xiphinema americanum in soybeans, and Pratylenchus coffeae in strawberries.
Other examples include the genera Belonolaimus and Longidorus, which are ectoparasites that feed deep within the roots using their long stylets, while those of the genus Helicotylenchus feed on the exterior of the root or partially burrow into the root to feed using its short, stout stylet. Most plant parasitic nematodes are soil-borne root pathogens, but a few species feed primarily upon shoot tissues. The majority of plant parasitic nematode species are in the class Chromodorea, order Rhabditida (formerly placed in the order Tylenchida). There are also nematodes feeding on algae, bacteria, fungi, insects, or even smaller nematodes.
Additional examples of plant nematodes include the pinewood nematode (Bursaphelenchus xylophilus), a nematode that infects pine trees; the root-knot nematodes (Meloidogyne spp.), the most destructive to soybean plants, causing “galls” on roots; the soybean cyst nematode (Heterodera glycines), which has been a problem throughout Arkansas’s history; stem and bulb nematodes (Ditylenchus spp.); and foliar nematodes of the genus Aphelenchoides, which includes a relatively new species of roundworm in Arkansas that surfaced in the late twentieth century and mainly attacks cotton plants. The effects of reniform nematodes on soybean crops are still unknown. In Arkansas, cotton is primarily affected by root-knot and reniform nematodes. Of these, root-knot nematodes cause billions of dollars of losses to numerous crop plants all over the world, making them the most commonly studied group of plant-parasitic nematodes on Earth. The control and treatment of plants infected with plant nematodes requires a combination of methods in an integrated pest management system. Few nematicides are available for use in soybeans; generally, nematodes can be managed much more economically through the use of resistant cultivars and crop rotation.
The Division of Agriculture of the University of Arkansas is the statewide research and extension agency serving Arkansas agriculture, and studies regarding plant nematodes are often done within this entity. In addition, the Arkansas Nematode Diagnostic Clinic is located at the Southwest Research and Extension Center in Hope. The primary purpose of the clinic is to provide nematode analyses for commodities that are either known or believed to have a problem with one or more species of plant-parasitic nematodes.
Significant Nematodes
One very important roundworm in biomedical research is the bacterial-feeding nematode, Caenorhabditis elegans, one of the best-understood invertebrates. The fate of every cell in C. elegans’s development has been carefully mapped; it was also the first animal to have its DNA sequence completely deciphered, and it is amenable to genetic analyses. The study of C. elegans has led to new insights into the details of animal development, behavior, and neurobiology, and has been of great value in biomedical research.
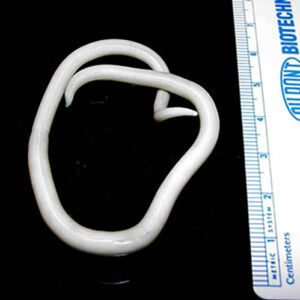
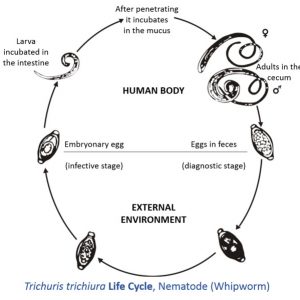
The order Trichurida includes six families and about sixty to seventy known species of whipworms, and the most important to humans and other primates is Trichurus trichiura. Pathology caused by this worm includes anemia, diarrhea, finger clubbing, growth retardation, and prolapsed rectum. Secondary bacterial infections can occur due to contamination of lesions produced by these worms. Another important worm in this order is the world’s largest intracellular parasite, Trichinella spiralis. Its larva resides in skeletal muscle fibers of wild and domestic animals, including humans. In the life cycle, twelve hours to two days after ingestion of raw or undercooked pork, female nematodes begin to penetrate the gut and secrete waste products. Inflammation, pain, diarrhea, fever, nausea, sweating, respiratory difficulty, and red blotches on the skin of the host may occur. Facial edema and fever occurs five to seven days post-infection due to worm waste products. During larval migration, blood vessel damage occurs resulting in edema, petechia, pneumonia, encephalitis, pleurisy, meningitis, nephritis, deafness, peritonitis, eye damage, and eventually brain damage. Occasionally death occurs due to myocarditis, local necrosis, and inflammation of cardiac muscles. About the ninth or tenth day post-infection, juveniles begin penetrating skeletal muscle fibers. Muscle pain, difficulty in swallowing, difficulty in breathing, weakened pulse, and low blood pressure can occur, which can result in heart damage and swelling of the esophagus and masseter muscles. This can lead to respiratory failure because of damage to the diaphragm and intercostal muscles.
The giant kidney worm Dioctophyme renale (order Dioctophymatida) is generally a parasite of wild mustelids, especially minks (Mustela vison). Reported in canids, bears, raccoons, otters, and seals, it is also found in domestic animals such as horses, cattle, and swine. It is even found in humans. In Arkansas, D. renale has been reported in northern river otters, Lontra canadensis. Generally, animals are infected with only one to three worms at a time.
Another dioctophymatid is in the genus Eustrongylides. There are about eleven to twelve species that live in hyperplastic tumors in the wall of the proventriculus of fish-eating birds. Larvae are found in aquatic oligochaetes, and if eaten by a fish, they migrate across the gut and encapsulate in its viscera and on the intestinal serosa. Various fishes, amphibians, and a few reptiles serve as second intermediate hosts. There are recent reports of Eustrongylides sp. in some vertebrates of Arkansas.
The order Mermithida contains numerous nematodes that are free living as adults but whose larval stages are parasitic in invertebrates (especially arthropods). Free-living adults do not feed and have no gut (they utilize a food storage organ for survival, termed a trophosome). Generally, the nematode consumes the arthropod during development and usually kills it during emergence. Most emerge when the infected arthropod contacts an aqueous medium.
The order Rhabditida (family Rhabdiasidae) includes Rhabdias bufonis and R. ranae, parasites in the lungs of frogs and toads worldwide. In the Western Hemisphere (including Arkansas) there may be several other Rhabdias species in amphibians, including salamanders. It has a heterogonic life cycle with a free-living generation interspersed between parasitic generations. The parasitic individual is a functional male before it becomes a female. Larvae penetrate the skin of an anuran and lodge in various tissues; all die except those that reach lungs. In the lungs, they mature into hermaphroditic adults. DNA sequencing methods are needed to unravel species of Rhabdias in amphibians of the state.
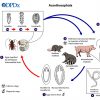
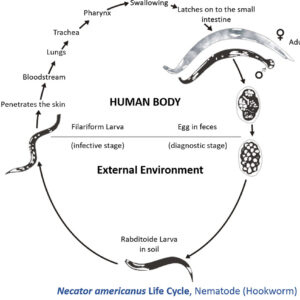
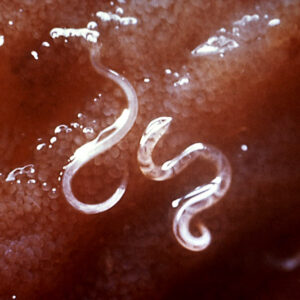
The order Strongylida (the bursate nematodes) includes the hookworms (superfamily Ancylostomoidea, family Ancylostomatidae) found in the small intestine of mammals, predominately carnivores and omnivores, with a few in herbivores. These nematodes have large, highly sclerotized buccal capsules with cutting teeth or plates. Adults are found in the gut of hosts, attached to the mucosa of the small intestine where they suck blood and tissue fluids. Representative species of medical or veterinary importance include Ancylostoma caninum (the most common hookworm of domestic canids and a common cause of cutaneous larval migrans or creeping eruption) and Necator americanus (in humans worldwide). Worldwide, hookworm infections number about thirteen billion with around 70,000 deaths yearly. Mebendazole is the drug most commonly used for treating hookworm disease. In Arkansas, the Rockefeller Sanitary Commission undertook a significant effort at eradicating hookworm infestation in people in the early 1910s; before this, some twenty percent of the population was believed to harbor hookworms.
The superfamily Trichostrongyloidea is very large, with fourteen to fifteen families. Unlike in hookworms and strongylids, the buccal capsule is absent or greatly reduced, the lips and a corona radiata are absent or vestigial, and teeth are rare. It is a highly diverse group, with many in the stomach and intestine of terrestrial vertebrates (especially mammals and birds). Some important species include Haemonchus contortus (the abomasal barber pole or twisted stomach worm of ruminants and cervids), Nippostrongylus braziliensis (in the small intestine of Rattus spp.), and Oswaldocruzia pipiens (in intestine of frogs), the latter of which has been reported in amphibians and reptiles in Arkansas.
The superfamily Metastrongyloidea (lungworms) includes seven families and about forty-five genera. All species infect mammals, and, as adults, most species occupying the lungs of the host are found in veins distant from the host or in pulmonary or mesenteric arteries with a few in frontal sinuses. Three important species found in game animals are Parelaphostrongylus andersoni and P. odocoilei, which are found in the blood vessels of the muscles of white-tailed deer (P. andersoni) and mule and black-tailed deer (P. odocoilei), and P. tenuis, which is found in the meninges of white-tailed deer, causing neurologic disease in reindeer, caribou, elk, moose, mule and black-tailed deer.
The Family Ascarididae is composed of species parasitic in terrestrial hosts with some growing to forty-five centimeters or more. Transmission may require terrestrial invertebrates or small mammals as paratenic or intermediate hosts. Some species have eggs directly infective for the definitive hosts. One important species is Ascaris suum (similar to species A. lumbricoides in humans) with swine as definitive hosts. Contamination by dirt, soiled fingers, and toys put in children’s mouths is a typical means of infection with A. lumbricoides. Ascariasis is thought to be in epidemic proportions in the southeastern United States, and worldwide 1.27 billion people are infected. Another member of this family is Baylisascaris procyonis with raccoons (Procyon lotor) and sometimes canids as definitive hosts. In these hosts, larvae migrate through the body, often causing extensive damage including neurological (CNS) problems. More than ten cases of fatal CNS disease have been recorded from children, and this is most likely a small fraction of the true number of cases. Mortalities of some mammals in zoos are the result of raccoons creating communal defecation sites, called latrines, on zoo properties and spreading the parasites.
The superfamily Heterakoidea is made up of intestinal parasites with a prominent preanal sucker surrounded by a cuticularized ring with most species found in birds, and some in amphibians and reptiles. One important species in the poultry industry, Heterakis gallinarum (Family Heterakidae), is a cecal nematode of domestic chickens, turkeys, and related birds. Eggs are picked up by arthropods and then can infect chickens when the bird ingests an arthropod with eggs. It has been reported from Arkansas in eastern wild turkeys (Meleagris gallolpavo silvestris). Although this parasite is not highly pathogenic in itself, it can be effectively eliminated using mebendazole or by raising birds on hardware cloth where they are not allowed to roam freely.
The order Oxyurida, with eight families (including pinworms) has more than 850 known species. It is the only major nematode group with adult representatives in both invertebrate and vertebrate hosts. The notable pinworm of humans, Enterobius vermicularis (family Oxyuridae), is also found in chimpanzees and baboons. It has been in Homo sapiens since the time of humanity’s origin in Africa and infects at least 400 million people (mostly children). Pinworms exhibit haplodiploidy, where males are haploid and arise by parthenogenesis via unfertilized eggs and females are diploid and develop from fertilized eggs. This results in a high degree of inbreeding. Pinworm infection (termed enterobiasis) is sometimes asymptomatic, but when symptoms do occur, they include anal itching, restlessness, and irritability, especially in children. Although pinworms are often found in the appendix, only rarely do they cause appendicitis. Occasionally, ectopic migration of gravid females from the anal region may occur. This can include infections in the uterus and fallopian tubes, uterine granulomas, invasion of the ovary and, rarely, worms in the peritoneum. Treatments include highly effective antihelminthic therapies such as albendazole, mebendazole, and pyrantel pamoate. However, since this nematode is easily transmitted among hosts without the need of an intermediate host, all individuals in a family with an affected family member must take the medication.
More surveys of fish parasites of Arkansas have been undertaken in the twenty-first century, and from them several nematodes have been reported, including some from two endemic species Noturus lachneri (Ouachita madtom) and Noturus taylori (Caddo madtom), and other fishes such as Cottus carolinae (banded sculpin), three species of Fundulus (topminnows), and various other species. Several nematodes have been documented from amphibians (frogs, toads, and salamanders) and reptiles (lizards and snakes) of the state, including new hosts and new distributional records for Arkansas being documented in several publications. However, fewer studies have been done on nematodes of birds (there is one on common grackles and eastern turkeys) of the state as well as little research on mammalian nematodes (there are two on bats and on P. lotor). Several new species in the genus Longidorus, which belong to a group of soil-inhabiting, migratory plant-parasitic nematodes, were found in surveys of longidorids of Arkansas.
For additional information:
Anderson, Roy C. Nematode Parasites of Vertebrates. Their Development and Transmission. Wallingford, UK: CAB International, 1992.
Chen, Z. X., and D. W. Dickson. Nematology: Advances and Perspectives. Vol. 1: Nematode Morphology, Physiology and Ecology. Wallingford, UK: CAB International, 2004.
Cox, Frank E. G. “History of Human Parasitology.” Clinical Microbiology Reviews 15 (2002): 595–612.
Crompton, David W. T. “How Much Human Helminthiasis Is There in the World?” Journal of Parasitology 85 (1999): 397–403.
Escalante Ortiz, Lucia Emperitriz. “Nematode Populations as Affected by Residue and Water Management in a Long-Term Wheat-Soybean Double Crop in Eastern Arkansas.” MS thesis, University of Arkansas, 2019. Online at https://scholarworks.uark.edu/etd/3467/ (accessed July 6, 2022).
Johnson, Arthur A. “Helminths of Common Grackles (Quisculus quiscula-versicolor, Vieillot) in Central Arkansas.” Proceedings of the Arkansas Academy of Science 38 (1984): 53–55. Online at http://libraries.uark.edu/aas/issues/1984v38/v38a13.pdf (accessed June 21, 2017).
Khanal, Churamani. “Identification of Root-Knot Nematodes (Meloidogyne spp.) of Arkansas Using Molecular Diagnostics.” MS thesis, University of Arkansas, 2014. Online at https://scholarworks.uark.edu/etd/2092/ (accessed July 6, 2022).
Kuzmin, Yuriy, Roman Svitin, Chris T. McAllister, Laura Guderyahn, and Vasyl V. Tkach. ”Descriptions and Phylogenetic Affinities of Two New Species of Rhabdias Stiles and Hassall, 1905 (Nematoda: Rhabdiasidae) from North American Frogs: Are We Still Only Scratching the Surface?” Journal of Parasitology 110.4 (2024): 339–350.
Lambert, Kris, and Sadia Bekal. “Introduction to Plant-Parasitic Nematodes.” The Plant Health Instructor 2002. http://www.apsnet.org/edcenter/intropp/PathogenGroups/Pages/IntroNematodes.aspx (accessed June 21, 2017).
Lee, D. L. The Biology of Nematodes. Taylor & Francis; New York, 2002.
Matute, M. M., P. W. Perschbacher, and A. Newell. “Determination of Benthic Soil Conditions Using Nematodes: Nematode Food Web Conditions of Fish Ponds in the Lincoln and Desha Counties of Arkansas.” Journal of the Arkansas Academy of Science 63 (2009): 131–138. Online at http://libraries.uark.edu/aas/issues/2009v63/v63a14.pdf (accessed June 21, 2017).
Mehlhorn, Heinz, ed. Encyclopedia of Parasitology. 4th ed. New York: Springer International Publishing, 2016.
McAllister, Chris T., Charles R. Bursey, and Angela D. Burns. “Gastrointestinal Helminths of Rafinesque’s Big-Eared Bat, Corynorhinus rafinesquii (Chiroptera: Vespertilionidae), from Southwestern Arkansas, U.S.A.” Comparative Parasitology 72 (2005): 121–123.
McAllister, Chris T., Charles R. Bursey, and Matthew B. Connior. “Helminth Parasites of the Dwarf American Toad, Anaxyrus americanus charlesmithi (Anura: Bufonidae), from Arkansas and Oklahoma.” Proceedings of the Oklahoma Academy of Science 94 (2014): 51–58.
McAllister, Chris T., Charles R. Bursey, Matthew B. Connior, Lance A. Durden, and Henry W. Robison. “Helminth and Arthropod Parasites of the Ground Skink, Scincella lateralis (Sauria: Scincidae), from Arkansas and Oklahoma.” Comparative Parasitology 81 (2014): 210–219.
McAllister, Chris T., Charles R. Bursey, Matthew B. Connior, Henry W. Robison, and Thomas J. Fayton. “Helminth Parasites of the Dark-Sided Salamander, Eurycea longicauda melanopleura (Caudata: Plethodontidae), from Arkansas.” Comparative Parasitology 82 (2015): 306–311.
McAllister, Chris T., Charles R. Bursey, William F. Font, Henry W. Robison, Matthew B. Connior, Donald G. Cloutman, and Thomas J. Fayton. “Helminth Parasites of the Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Interior Highlands of Arkansas.” Journal of the Arkansas Academy of Science 69 (2015): 135–138. Online at http://libraries.uark.edu/aas/issues/2015v69/v69a24.pdf (accessed June 21, 2017).
McAllister, Chris T., Charles R. Bursey, William F. Font, and Henry W. Robison. “Helminth Parasites of the Banded Sculpin, Cottus carolinae (Scorpaeniformes: Cottidae), from Northern Arkansas, USA.” Comparative Parasitology 81 (2014): 203–209.
McAllister, Chris T., Charles R. Bursey, William F. Font, Henry W. Robison, Stanley E. Trauth, Donald G. Cloutman, and Thomas J. Fayton. “Helminth Parasites of the Northern Studfish, Fundulus catenatus (Cypriniformes: Fundulidae) from the Ouachitas and Ozarks of Arkansas, U.S.A.” Comparative Parasitology 83 (2016): 78–87.
McAllister, Chris T., Charles R. Bursey, Thomas J. Fayton, Donald G. Cloutman, Henry W. Robison, Matthew B. Connior, and Stanley E. Trauth. “Helminth Parasites of the Blackstripe Topminnow, Fundulus notatus (Cyprinidontiformes: Fundulidae), from Arkansas and Oklahoma, U.S.A.” Comparative Parasitology 83 (2016): 227–236.
McAllister, Chris T., Charles R. Bursey, Thomas J. Fayton, Henry W. Robison, and Stanley E. Trauth. “New Host and Geographic Distributional Records for Eustrongylides sp. (Nematoda: Dioctophymatoidea: Dioctophymatidae) from Eight Vertebrates from Arkansas, Oklahoma and Texas.” Proceedings of the Oklahoma Academy of Science 95 (2015): 26–32.
McAllister, Chris T., Charles R. Bursey, Henry W. Robison, David A. Neely, Matthew B. Connior, and Michael A. Barger. “Miscellaneous Fish Helminth Parasite (Trematoda, Cestoidea, Nematoda, Acanthocephala) Records from Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 78–86. Online at http://libraries.uark.edu/aas/issues/2014v68/v68a12.pdf (accessed June 21, 2017).
McAllister, Chris T., Charles R. Bursey, and Stanley E. Trauth. “New Host and Geographic Distribution Records for Some Endoparasites (Myxosporea, Trematoda, Cestoidea, Nematoda) of Amphibians and Reptiles from Arkansas and Texas, U.S.A.” Comparative Parasitology 75 (2008): 241–254.
McAllister, Chris T., Matthew B. Connior, Charles R. Bursey, and Henry W. Robison. “A Comparative Study of Helminth Parasites of the Many-Ribbed Salamander, Eurycea multiplicata and Oklahoma salamander, Eurycea tynerensis (Caudata: Plethodontidae), from Arkansas and Oklahoma.” Journal of the Arkansas Academy of Science 68 (2014): 87–96. Online at http://libraries.uark.edu/aas/issues/2014v68/v68a13.pdf (accessed June 21, 2017).
McAllister, Chris T., Steve J. Upton, and Charles R. Bursey. “Parasites (Coccidia, Trematoda, Nematoda) from Selected Bats of Arkansas.” Journal of the Arkansas Academy of Science 58 (2004): 133–136. Online at http://libraries.uark.edu/aas/issues/2004V58/v58a22.pdf (accessed June 21, 2017).
Ortiz, Lucia Emperatriz Escalante. “Nematode Populations as Affected by Residue and Water Management in a Long-Term Wheat-Soybean Double Crop in Eastern Arkansas.” MS thesis, University of Arkansas, 2019.
Perry, R. N., and M. Moens. Plant Nematology. Wallingford, UK: CAB International: 2006.
Poinar, George O. The Natural History of Nematodes. Englewood Cliffs, NJ: Prentice Hall, 1983.
Richardson, Dennis J. “A Survey of the Helminth Parasites of the Raccoon (Procyon lotor) from North Central Arkansas.” MS thesis, University of Central Arkansas, 1990.
Thogmartin, Wayne E., James E. Johnson, Bradley A. Schaeffer, and Camille C. Ciriano. “Survey of Diseases in Wild Turkeys.” Journal of the Arkansas Academy of Science 53 (1999): 114–119. Online at http://libraries.uark.edu/aas/issues/1999v53/v53a19.pdf (accessed June 21, 2017).
Thomas, Andrew L., Robert T. Robbins, R. Andrew Allen, Terry Kirkpatrick, Elijah A. Bergmeier, Juan Cabrera-Garcia, Jackie L. Harris, and R. Keith Striegler. “Distribution of Plant-parasitic Nematodes in Missouri and Arkansas, USA, Vineyards.” HortScience, 60, no. 11 (2025): 1903–1907. Online at https://doi.org/10.21273/HORTSCI18858-25 (accessed September 29, 2025).
Wehunt, E. J., A. M. Golden, and R. T. Robbins. “Plant Nematodes Occurring in Arkansas.” Journal of Nematology 21 (1989): 677–681.
Weingartz, Christine Rose. “A Study of the Prevalence of Gastrointestinal Nematodes in Goats Obtained from Northwest Arkansas.” MS thesis, University of Arkansas, 2017. Online at https://scholarworks.uark.edu/etd/2587/ (accessed July 6, 2022).
Ye, Weimin, and R. T. Robbins. “Longidorus grandis n. sp. and L. paralongicaudatus n. sp. (Nematoda: Longidoridae), Two Parthenogenetic Species from Arkansas.” Journal of Nematology 35 (2003): 375–387.
———. “Longidorus paravineacola n. sp. (Nematoda: Longidoridae), a New Species from Arkansas.” Journal of Nematology 35 (2003): 388–394.
———. “Longidorus biformis n. sp. and L. glycines n. sp. (Nematoda: Longidoridae): Two Amphimictic Species from Arkansas.” Journal of Nematology 36 (2004): 1–13.
Chris T. McAllister
Eastern Oklahoma State College








Comments
No comments on this entry yet.