calsfoundation@cals.org
Trematodes
aka: Flatworms
aka: Flukes
Trematodes (flukes) include parasitic flatworms belonging to the phylum Platyhelminthes, class Trematoda, and subclasses Aspidogastrea (two orders, four families) and Digenea (ten orders, more than seventy-two families). The class numbers between 18,000 and 24,000 species; they are found primarily in a variety of animals, including humans and other vertebrates. Modern phylogenetic analysis reveals that the worms of class Monogenoidea (monogenetic flukes) are no longer included within the Trematoda and are more closely related to tapeworms. The modern mobility of human beings, combined with the international transportation of animals and foodstuffs that can be infected, means that diagnoses can occur well outside the areas where trematode species are endemic. However, while trematodes do occur in Arkansas, they do not pose a great health risk, as they primarily occur in species of animals not commonly eaten by humans.
One small group of trematodes belongs to the subclass Aspidogastrea (sometimes referred to as Aspidobothrea), which includes about eighty species. They are parasites of mollusks but can also infect fishes (including both teleost and cartilaginous species) and turtles. Specific cartilaginous hosts of aspidogastreans include chondrichthyan fishes (sharks, rays, and chimaeras), a group that is 450 million years old, whereas those of the sister group of the aspidogastreans, the digeneans, are known from both condrichthyan and teleost fishes (210 million years old) as well as from various “higher” vertebrates.
Species range in length from less than one millimeter to over several centimeters. Aspidogastreans have a less complex life cycle than their digenean relatives, and reproduction results in only one egg. These eggs are laid and passed out of the host with the host’s feces, and in some, such as Amphilina foliacea from sturgeon, the eggs do not hatch until they are eaten by the amphipod intermediate host. In others, such as Austramphilina elongata from the long-necked turtle (Chelodina longicollis) in southeastern Australia, they hatch in the water and the larvae swim around until they are able to infect a suitable, or sometimes unsuitable, host. In some species—such as Aspidogaster conchicola from freshwater mussels and Lobatostoma manteri from the snubnosed dart (Trachinotus blochi) of the Great Barrier Reef—the eggs are not laid until the larvae are nearly ready to hatch. These larvae can be either ciliated (having hairlike projections), in which case they normally have two rings of cilia (i.e., Multicotyle purvisi from freshwater snails and turtles in southeastern Asia and Cotylogaster occidentalis from freshwater drum, Aplodinotus grunniens), or they can be unciliated as in Rugogaster hydrolagi from rectal glands of holocephalan fishes and Multicalyx elegans from the intestine of holocephalans and elasmobranchs.
In the intermediate host, the larvae grow a certain amount and then wait until the intermediate host is eaten by the primary host. Interestingly, aspidogastreans may survive for many days or perhaps even weeks outside a host in water or saline solution. This evidence has led some to propose that aspidogastreans are archaic trematodes, not yet well adapted to specific hosts, that have given rise to the more “advanced” digenean trematodes. They further propose that the complex life cycles of digenean trematodes have evolved from the unsophisticated ones of aspidogastreans. However, because none of these aspidogastreans have medical or economic importance, they attract little attention compared to the Digenea.
The more common flukes of the subclass Digenea are mostly dorsoventrally flattened (flattened on the top and bottom surfaces) and possess a muscular oral sucker that surrounds the mouth. In addition, most also possess a midventral or posterior acetabulum (ventral sucker) used for locomotion and attachment to a host. The digestive tract normally consists of a short esophagus (often surrounded by a muscular pharynx), which then often (but not always) splits into a pair of blind intestinal cecae. Generally, host tissues are drawn into the oral sucker and are then digested by the strong pumping action of the pharynx. However, in the schistosomes (Schistosoma spp.), which live in the blood vessels and ingest blood, a pharynx is not present.
Most trematodes are hermaphroditic (having reproductive organs associated with both male and female sexes), except the schistosomes, and many trematodes self-fertilize. The male reproductive system usually consists of two testes (range from one to several hundred), each of which may have a vas efferens that connects to form a common duct, the vas deferens. The vas deferens leads to the genital pore, which usually has associated structures such as an internal seminal receptacle for sperm storage, a prostate gland that may add secretions to the sperm, and a cirrus pouch in some, the so-called male copulatory organ.
The female reproductive system is more complicated and consists of a single ovary, an oviduct, a seminal receptacle in some for sperm storage, vitelline glands along the lateral margins of the body that provide material for egg shell formation, and a series of glandular structures that aid in egg shell maturation (e.g., Lauer’s canal, which functions as a vagina, along with the Mehlis gland and ootype). Females may also have a uterus which may be filled with eggs, and perhaps a modification of the end of the uterus called a metraterm. The body of a fluke is covered by a living layer of cells called a tegument, which functions in nutrient absorption. Thus, flukes can digest and absorb nutrients not only across the gut wall but also across the outer body. Ornamentation, such as cuticular spines, papillae, or tubercles, is often present along the tegument and can often be seen by using simple light microscopy.
Flukes can be loosely categorized six ways based on the location of suckers. The following are examples: (1) a monostome has only an oral sucker as in the genus Cyclocoelum, (2) an amphistome (genus Zygocotyle) has an oral sucker plus an acetabulum (ventral sucker) at the posterior end of the body, (3) a distome has an oral sucker and a ventral sucker, although the latter is located somewhere other than the posterior end as in the genus Alloglossidium, (4) a holostome (genus Cyathocotyle) is a type of distome that has the body split into distinct anterior and posterior portions, (5) an echinostome has long tegumental spines surrounding the oral sucker as in Echinostoma, and (6) a schistosome has a strong oral sucker with an acetabulum near the anterior end, as in the medically important genus Schistosoma.
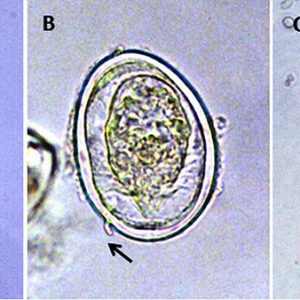
The life cycle of a digenean trematode is indirect and often involves two or more hosts. However, there is a species opecoelidae fluke in the genus Plagioporus that matures in snails. Eggs of most species are operculate (with a lid), except the schistosomes, and laid either embryonated or unembryonated (with or without an embryo). The eggs of some species hatch in water, while those of others require ingestion by the appropriate intermediate host. Once the egg hatches, however, a ciliated larva called a miracidium emerges and penetrates the tissue of the intermediate host (usually a snail or clam) with anterior penetration glands. Depending upon the species, the miracidium may develop into a sporocyst (an asexual reproductive structure without mouth or intestine that may give rise to daughter sporocysts, rediae, or cercariae) or redia (an asexual reproductive structure with mouth and gut that may give rise to daughter rediae or cercariae).
Whichever phases of asexual development a species possesses, the final outcome of asexual multiplication is tailed cercariae in some, which aid them in swimming; however, some have rudimentary tails or none at all. Depending upon the species, these sexually immature (juvenile) forms may do one of several things. Some species encyst on vegetation as metacercariae and remain dormant until eaten by an appropriate host. Some penetrate the skin or muscle tissue of an animal (especially fishes) and remain dormant as metacercariae until the fish is eaten by the final (definitive) host. Others may penetrate the skin of the definitive host directly such as the schistosomes, thus forming no metacercaria. Once inside the final vertebrate host, the cercaria casts off its tail, migrates to its target organ(s), and finally matures into an adult worm.
One interesting digenean (belonging to the genus Halipegus) is found in frogs and has two different hosts involving several generations. Mollusks, copepods, ostracods, or dragonfly nymphs are typically the first intermediate hosts, whereas frogs can be second intermediate or definitive hosts. This parasite possesses a non-encysted metacercariae. Embryonated eggs are passed in the feces of the frog, hatch when eaten by a snail, and develop into cercariae, which are swallowed by copepods. The infected cyclopoid copepods are swallowed by tadpoles. The worms remain in the mesenteries until the frog metamorphosizes, at which time the parasite migrates up to the esophagus into the eustachian tubes.
A number of medically important digenean flukes are found in humans and other animals. One is Dicrocoelium dendriticum that lives in the bile ducts of mammals, especially cattle and deer, and is distributed in Europe and, following its introduction, portions of the northeastern United States. Humans are considered only accidental hosts. The salmon fever fluke, Nanophyetus salmincola, which lives in the intestine of many mammals, occurs along the northwestern coast of North America and Siberia. This species is known to infect over thirty different species of fish-eating mammals and occurs in the crypts (glands) of the small intestine of canids, felids, mustelids, bears, and humans; some birds may also harbor adult worms. This worm has been reported in North America on at least ten occasions, and a similar species, N. schikhobalowi, infects up to ninety-eight percent of humans in Siberia.
The group of digeneans collectively called “heterophyids” contains more than fifty species worldwide; these species are capable of infecting humans. One common species in this group is Metagonimus yokagawi of fish-eating mammals in the Far East, the former Soviet Union, and the Balkans. Patients infected with M. yokogawai present with mucous, diarrhea, and vague abdominal symptoms. Prognosis is usually good, except in cases of embolization (blocked blood vessels). Another heterophyid species, Heterophyes heterophyes, is a small fluke first discovered as a human infection in Cairo, Egypt, in 1851. It has also been reported from China, Korea, Japan, Taiwan, and the Philippines. Heavy infections, which are not uncommon, cause damage to the host’s mucosa and produce intestinal pain and mucous diarrhea. If eggs enter the bloodstream, patients can develop heterophyid myocarditis, reported in fifteen percent of patients with cardiac failure in the Philippines. In addition, eggs in the brain or spinal cord can lead to neurological disorders that are sometimes fatal.
Another group, schistosomes, consists of slender and elongate, blood-vascular worms, with separate sexes and very anterior suckers. These worms cause the serious human disease known since antiquity as schistosomiasis. The female worm lies within the gynecophoral longitudinal groove (canal) in the ventral surface of the male. Schistosomes are responsible for 240 million human infections and up to 200,000 deaths per year. Schistosoma haematobium lives principally in veins of the urinary bladder plexus of people from India, the Middle East, and Africa, and the pathogenesis is almost entirely due to the eggs and not the adult worms. In chronic disease, eggs become trapped in the bladder wall, resulting in the formation of granulomata. Following prolonged infection, the ureters may become obstructed and the bladder may become thickened, resulting in abnormal urinary function, urinary infection, and damage to the kidneys. Another famous schistosome species is Manson’s blood fluke, Schistosoma mansoni, so named for Sir Patrick Manson (1844–1922), Scottish physician, who worked with the fluke in 1905.
The sheep liver fluke, Fasciola hepatica, one of the largest flukes in the world (30 × 13 millimeters) infects a variety of mammals, especially the bile duct (and occasionally gallbladder) of ruminants worldwide; humans are sometimes infected. These human infections have been reported in Europe, northern Africa, Cuba, and South America. These flukes are capable of causing considerable damage to their host as they migrate through the liver tissue, or by blocking bile ducts. The giant intestinal fluke, Fasciolopsis buski, is the most common human intestinal trematode, an elongate and oval species reaching lengths of twenty to seventy-five millimeters and widths up to twenty millimeters; it infects the small intestine of swine and humans in Asia. In 1947, there were an estimated ten million human infections, and the number is likely greater today. Heavy infections can block the passage of food and interfere with normal digestive functions, and long-term infections can lead to ulceration, hemorrhage, intestinal abscesses, and death.
The Chinese liver fluke, Clonorchis sinensis, infects the bile duct of a variety of fish- eating mammals, including humans, swine, canids, felids, rodents, lagomorphs, and camelids in Asia and southeast Asia. This fluke has a wide geographic range including China, Korea, Japan, Taiwan, and Vietnam. Infection outside the natural range of C. sinensis involves humans acquiring the worm by eating frozen, dried, or pickled fish from endemic areas. In New York City, for example, prevalence of infection with clonorchiasis was twenty-six percent among immigrant Chinese. The first intermediate host is a variety of snail species (most common is Parafossarulus manchouricus) where sporocyst and redial generations occur. The metacercariae develop within the musculature of a variety of fish as second intermediate hosts, predominately cyprinid fishes, including grass carp (Ctenopharyngodon idella), considered a Cantonese delicacy.
In birds, the oviduct fluke, Prosthogonimus macrorchis, is found in the oviducts, bursa of Fabricus, intestine, and cloaca of North American anseriform, galliform, and passeriform birds. The first intermediate host is snails of the genus Amnicola, and metacercariae develop within dragonfly nymphs after being pulled into their rectal branchial chamber. In North America, the prevalence of P. macrorchis is declining because chickens are often maintained in confined spaces and, thus, no longer able to pursue dragonflies as prey. Kellicott’s lung fluke, Paragonimus kellicotti, is found encysted in lung granulomas of a variety of mammals in North America east of the Rocky Mountains, including humans, canids, felids, mustelids, opossums, raccoons, swine, and ruminants. The first intermediate is the snail, Pomatiopsis lapidaria, and metacercariae encyst on the viscera, especially the heart muscle, of the second intermediate hosts: crayfish of the genus Cambarus and water crabs. These flukes cause an inflammation in the lungs and connective tissue proliferation. In addition, many worms migrate to sites other than the lungs and can cause serious medical problems.
In Arkansas, several digenean trematodes are commonly encountered in various vertebrates when necropsied (examined after death). Adult Posthodiplostomum minimum occur especially in the small intestine of fish-eating birds in the orders Ciconiiformes (wading birds) and Charadriiformes (shorebirds) and a wide range of other birds, mammals, and even reptiles and amphibians. First intermediate hosts are snails of the genus Physa, and metacercariae termed “white grub” can be found encysted within the viscera of fish, especially centrarchids (sunfish, crappie, and bass) and also cyprinids (minnows and shiners).
Another relatively common digenean trematode found in both game and non-game Arkansas fishes statewide are several species of strigeoids that cause black spots in the skin. These include species of Neascus and Uvulifer ambloplitis. The host fish responds to the neascus (metacercariae) in its skin by deposition of melanin pigment. The result is a conspicuous black spot (sometimes many spots) indicating presence of the infection, and when the definitive host (likely a kingfisher) ingests a host fish, the definitive host becomes infected with a fluke that matures in about a month. When fishermen encounter fishes with “black-spot,” they often discard them as diseased.
Other common Arkansas digeneans include the clinostomes (primarily Clinostomum marginatum), the adult of which resides in the mouth and esophagus of reptiles and, especially, fish-eating birds (e.g., herons and bitterns). Eggs come into water with the feces, and the miracidia can penetrate planorbid snails. Sporocyst and redial growths then occur, creating cercariae that encyst as metacercariae throughout the body of second intermediate hosts such as fishes and amphibians (salamanders, frogs, and toads). The metacercariae are large and yellowish, so their presence is often termed “yellow grub disease.” Vertebrates that eat fish can become infected by eating second intermediate hosts containing the metacercariae. Clinostomes are very common in amphibians, fish, and fish-eating birds in Arkansas, and a great deal of research has been done on Clinostomum spp. in the state. Interestingly, some of the largest populations of clinostomes are in smallmouth bass (Micropterus dolomieu) of Crooked Creek in Marion County.
Although much has been reported on the trematode parasites of game fishes of the state, recent research reveals the incredible diversity of trematodes found in non-game fishes of Arkansas. Several new species have been discovered, including those in the genera Alloglossidium, Crepidostomum, Creptotrema, Homalometron, Lissorchis, and Plagioporus. Biologists using both morphometric (quantitative analysis of form) and molecular techniques will likely add to the growing knowledge of the trematodes of Arkansas and beyond.
For additional information:
Ashford, Richard W., and William Crewe. The Parasites of Homo sapiens. London: Taylor & Francis, 2003.
Boeger, Walter A., and Delane C. Kritsky. “Phylogeny and a Revised Classification of the Monogenoidea Bychowsky, 1937 (Platyhelminthes).” Systematic Parasitology 26 (1993): 1–32.
Cloutman, Donald G. “Parasite Community Structure of Largemouth Bass, Warmouth, and Bluegill in Lake Fort Smith, Arkansas.” Transactions of the American Fisheries Society 104 (1975): 277–283.
Cox, Frank E. G. “History of Human Parasitology.” Clinical Microbiology Reviews 15 (2002): 595–612.
Daly, James J., Sr. “Distribution of Yellow Grub (Clinostomum marginatum) Metacercariae in Black Bass (Micropterus spp.) from Arkansas Ozark and Ouachita Reservoir Lakes.” Journal of the Arkansas Academy of Science 67 (2013): 173–176. Online at http://libinfo.uark.edu/aas/issues/2013v67/v67a29.pdf (accessed December 23, 2025).
Eastburn, R. L., T. R. Fritsche, and C. A. Terhune Jr. “Human Intestinal Infection with Nanophyetus salmincola from Salmonid Fishes.” American Journal of Tropical Medicine and Hygiene 36 (1987): 586–591.
Fiedor, Taylor Michelle. “Montaine Molluscs: Factors Affecting Snails and Their Trematodes throughout the Mountain Regions of Arkansas.” MA thesis, Arkansas State University, 2024.
Gibson, David I. “Questions in Digenean Systematics and Evolution.” Parasitology 95 (1987): 429–460.
Hoffman, Glenn L. Parasites of North American Freshwater Fishes. 2nd ed. Ithaca, NY: Comstock Publishing Associates, 1999.
Littlewood, D. T. J., Klaus Rohde, and K. A. Clough. “Interrelationships of All Major Groups of Platyhelminthes: Phylogenetic Evidence from Morphology and Molecules.” Biological Journal of the Linnean Society 66 (1999): 75–114.
McAllister, Chris T., Charles R. Bursey, John A. Crawford, Andrew R. Kuhns, Christopher Shaffer, and Stanley E. Trauth. “Metacercariae of Clinostomum (Trematoda: Digenea) from Three species of Ambystoma (Caudata: Ambystomatidae) from Arkansas and Illinois, U.S.A.” Comparative Parasitology 77 (2010): 25–30.
McAllister, Chris T., Charles R. Bursey, Henry W. Robison, David A. Neely, Matthew B. Connior, and Michael A. Barger. “Miscellaneous Fish Helminth Parasite (Trematoda, Cestoidea, Nematoda, Acanthocephala) Records from Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 78–86. Online at http://libinfo.uark.edu/aas/issues/2014v68/v68a12.pdf (accessed December 23, 2025).
McAllister, Chris T., Charles R. Bursey, and Stanley E. Trauth. “New Host and Geographic Distribution Records for Some Endoparasites (Myxosporea, Trematoda, Cestoidea, Nematoda) of Amphibians and Reptiles from Arkansas and Texas, U.S.A.” Comparative Parasitology 75 (2008): 241–254.
McAllister, Chris T., Matthew B. Connior, William F. Font, and Henry W. Robison. “Helminth Parasites of the Banded Sculpin, Cottus carolinae (Scorpaeniformes: Cottidae), from Northern Arkansas, U.S.A.” Comparative Parasitology 81 (2014): 203–209.
McAllister, Chris T., William F. Font, Matthew B. Connior, Henry W. Robison, Thomas J. Fayton, Nicholas H. Stokes, and Charles D. Criscione. “Trematode Parasites (Digenea) of the Slender Madtom, Noturus exilis and Black River Madtom, Noturus maydeni (Siluriformes: Ictaluridae) from Arkansas, U.S.A.” Comparative Parasitology 82 (2015): 137–143.
McAllister, Chris T., William F. Font, Thomas J. Fayton, and Henry W. Robison. “Helminth Parasites of Select Cyprinid Fishes from the Red River Drainage of Southeastern Oklahoma.” Proceedings of the Oklahoma Academy of Science 94 (2014): 81–86.
McAllister, Chris T., Renn Tumlison, Henry W. Robison, and Stanley E. Trauth. “An Initial Survey on Black-Spot Disease (Digenea: Strigeoidea: Diplostomidae) in Select Arkansas Fishes.” Journal of the Arkansas Academy of Science 67 (2013): 200–203. Online at http://libinfo.uark.edu/aas/issues/2013v67/v67a33.pdf (accessed December 23, 2025).
Rohde, Klaus. “The Aspidogastrea, An Archaic Group of Platyhelminthes.” In Interrelationships of the Platyhelminthes, edited by D. T. J. Littlewood and Rodney A. Bray. New York: Taylor and Francis, 2001.
Schell, Stewart C. Trematodes of North America North of Mexico. Moscow: University Press of Idaho, 1985.
Tanaka H., and M. Tsuji. “From Discovery to Eradication of Schistosomiasis in Japan: 1847–1996.” International Journal of Parasitology 27 (1997): 1465–1480.
Chris T. McAllister
Eastern Oklahoma State College







Comments
No comments on this entry yet.