calsfoundation@cals.org
Aspidogastreans
aka: Aspidobothrians
aka: Aspidobothreans
An interesting group within the Class Trematodes (flukes) includes the Subclass Aspidogastrea. The Aspidogastrea is a very small taxon with around eighty species within thirteen genera, and their hosts include molluscs (sometimes spelled “mollusks”) and various vertebrates. They are of particular curiosity among parasitologists because of their unique structure, their simple life cycles (which may well be the most “primitive” ones among the trematodes), and the extraordinarily complex sensory/nervous systems found in some species.
There has been some debate among taxonomists about the relationships of the various genera of aspidogastreans; however, according to the prevalent view, there are four families as follows:
(1) Rugogastridae: with two caeca and single row of transverse rugae comprising a single genus Rugogaster; there are two species from the rectal glands of holocephalan ratfishes;
(2) Stichocotylidae: with one caecum and a single row of well separated suckerlets; includes a monotypic species (Stichocotyle nephropis) from the intestine of elasmobranchs;
(3) Multicalycidae: with one caecum and a single ventral row of alveoli separated by transverse septa); there is a single genus Multicalyx from the intestine of holocephalan fishes and elasmobranchs; and
(4) Aspidogastridae: with one caecum and a ventral disk with three or four rows of alveoli; it is the largest family with nine genera in three subfamilies from molluscs, turtles, and teleost fishes.
There are two orders, the Aspidogastrida with the single family Aspidogastridae, and the Stichocotylida composed of the other three families. The sister group of the Aspidogastrea is the larger trematode subclass the Digenea, with thousands of species and many families. Apparently, the ancestor of the Digenea split from the ancestor of the Aspidogastrea early in evolutionary history, probably greater than 400 million years ago during the Devonian Period. Comparative studies using molecular tools (18S), as well as extensive transmission electron microscopic (TEM) studies, have demonstrated that the Trematoda (Aspidogastrea + Digenea), the Eucestoda, Gyrocotylidea and Amphilinidea, as well as the Polyopisthocotylea and Monopisthocotylea, and all the major groups of parasitic Platyhelminthes, have one common ancestor.
Morphologically, larval aspidogastreans possess a posterior sucker as well as an anterior pseudo (or false) sucker that is not separated from the surrounding tissue by a genuine sheath of connective tissue. On the other hand, adults do not have a posterior or ventral sucker, but an adhesive (ventral) disk consisting of transverse grooves (rugae), a single row of well-separated small suckers (suckerlets), or three to four rows of alveoli (suckerlets) on a ventral disk. There is no multiplication of larval stages in the mollusc host (i.e., a single egg produces a single adult). In all aspidogastreans that have been examined to date, the posterior sucker of the larva is transformed into an adhesive disk. For example, in those in the genus Rugogaster, the rugae are formed by the posterior wall of the sucker. In species of the genera Lobatostoma and Multicotyle, among others, alveoli are formed within the sucker.
The life cycles of several species of aspidogastreans have been documented. There are two primary types: (1) where the entire life cycle can be completed in molluscs (freshwater bivalves or snails), although vertebrates can act as facultative (not obligate) hosts, and (2) in the other type, both a mollusc and a vertebrate are required for completion of the life cycle. The first example is what occurs in Aspidogaster conchicola, whose life cycle has been known for a long time, at least since the beginning of the nineteenth century. Adult worms generate eggs in which a non-ciliated larva with an anterior and posterior sucker develops. Molluscs become infected either by ingesting eggs containing larvae or by larvae that have already hatched. The life cycle is completed without involvement of a vertebrate host; however, if a fish ingests an infected mollusc, adults can produce eggs in this host. An additional example is Cotylaspis insignis, which have sexually mature specimens that are normally found as an ectoparasite at the junction of the gills and visceral mass of a variety of species of freshwater mussels and from some aquatic turtles. The second kind of life cycle occurs in Lobatostoma manteri from the small intestine of the marine teleost carangid snub-nosed dart fish, Trachinotus blochi. Adults inhabit the small intestine of the fish, where they possess fully mature male and female reproductive systems with one large posterior testis and a uterus filled with eggs that are shed through the gonopore at the anterior end. Ova are deposited in the feces by the fish host on the sea floor, where they are eaten by snails such as Clypeomorus batillariaeformis (=Cerithium [Clypeomorus] moniliferum). In the digestive gland of the snail, the posterior sucker of the larva develops to the adhesive disk, and reproductive organs develop to (almost) the final state, without, however, maturing and producing sperm and eggs. Interestingly, on the Great Barrier Reef of Australia at Heron Island, only juvenile Trachinotus were found to be infected with L. manteri. They crush the very thick-shelled snails between their well-developed pharyngeal plates. Larval parasites hatch in the stomach of the snails but move into the digestive gland to mature. Similar to L. manteri, Multicotyle purvisi also needs a mollusc and vertebrate host for the completion of its life cycle. However, infection of the mollusc is not by an egg that is ingested, but by a larva that hatches in freshwater. Larvae are inhaled by snails and migrate into their kidneys, where they grow to the stage infective to aquatic turtles.
Some other species of aspidogastreans have more complex life cycles. For example, larvae of Stichocotyle nephropis occur as encapsulations in the intestinal wall of lobsters, and the adults infect elasmobranchs. In addition, immature Multicalyx, adults of which infect holocephalans and elasmobranchs, have been recorded from the intestine of teleost fishes. These latter examples suggest that, in addition to the intermediate and definitive hosts, a further host acting as a transport host (i.e., a host containing immature stages which do not mature in it) may be involved.
The digestive system of aspidogastreans is simple. It consists of a funnel-like or, in some cases, a muscular sucker or one with several muscular lobes, a spheroidal pharynx, and a caecum near the posterior end of the organism.
The osmoregulatory system consists of flame cell protonephridia that feed into an excretory bladder near the posterior end of the body. A single (usually) excretory pore is dorso-subterminal or terminal.
The nervous system of the Aspidogastrea shows a greater complexity than that of turbellarians. In one aspidogastrean, Multicotyle, the number of anterior connectives (cerebral commissures) is much greater than in any other species of the many turbellarians that have been examined, and there are two rings of commissures, one close to the tegument, the other deeper in the tissue. The dorsal part of an interior commissure just anterior to the pharynx forms the brain. More posteriorly, the nerves form a typical system of connectives and commissures, as well as a complex pattern innervating the ventral (adhesive) disk. Interestingly, a dense network of nerve fibers (nerve plexus) innervates the intestine, and also the connective tissue septum separating the dorsal part of the body from the ventral disk. TEM of the nerves of M. purvisi shows a nerve sheath around parts of a posterior connective, a structure not reported from other flatworms. Larvae of M. purvisi and L. manteri possess sensory receptors that have been examined by several researchers. In the former, a total of thirteen receptor types are present, including a paired eye and a paired receptor complex dorsal to the mouth cavity. Each of these complexes consists of two dendrites, one forming a large liquid-filled cavity with at least ten short cilia lacking ciliary rootlets but possessing basal bodies and lamellate extensions of the ciliary membrane, the other penetrating the anterior wall of the cavity formed by the first dendrite and possessing a star-shaped, single cilium. Each ocellus (eye) consists of one pigment cell and two receptor cells with light-sensitive dendritic endings called rhabdomeres. The larva of L. manteri has only about nine types of receptors: eyes and anterior receptor complexes are not present. The difference between the two larvae is shown in the way they infect the intermediate host. The egg of the latter species does not hatch immediately; however, it is ingested by a snail, then hatches. It swims in water, is attracted to the surface layer by light stimuli, and is then inhaled by a snail host. The nervous system of larval Multicotyle shows the basic pattern also found in the adult, with nerves innervating the pharynx, intestine, and posterior sucker, and a large number of anterior connectives. The larva of M. purvisi has a larger variety of sensory receptors than that of L. manteri, including a pair of eyes and an anterior paired receptor complex that are absent in the latter species. It also has ten ciliary tufts and a coat of microfilia (very thin processes of the tegument). On the other hand, the larva of L. manteri has a well-developed pseudosucker absent in the former species. The adult of M. purvisi reaches a length of at least 10mm and that of L. manteri, about 7mm. The former species has a uterus coiled up in the anterior part of the body, with relatively few ova; the latter species has a uterus filling most of the body, with a large number of eggs. The juvenile and adult of both species have a large number of marginal organs (terminal parts of glands) between the marginal alveoli of the adhesive disk.
Like almost all trematodes (except the schistosomes), aspidogastreans are hermaphroditic. They are able to either self-fertilize or mate with another individual. For example, one species, M. purvisi, from the stomach and intestine of freshwater turtles in Southeast Asia, reaches a total length of about 10 mm and contains both a fully mature male as well as a fully mature and gravid female reproductive system. There may be one or more testes located posterior to the ovary. The female system consists of an ovary, vitelline cells, a uterus, and associated ducts. Unique characteristics of the Aspidogastrea include a septate oviduct (i.e., the oviduct carrying the egg cells from the ovary has a number of concentric constrictions) and the marginal bodies, which were long considered to be sensory in nature but are in fact secretory organs.
One of the more common aspidogastreans is A. conchicola, a parasite of freshwater bivalves. It occurs in the pericardial cavity of clams in Africa, Europe, and North America. Other hosts include other molluscs, fishes, and turtles.
In Arkansas, Lophotaspis interiora was described in 1931 from a single specimen taken from an alligator snapping turtle, Macrochelys temminckii, collected from a lake near the Black River at Cord (Independence County). This host association is of special interest because the turtle was kept in captivity for three months at Macon, Illinois, where it eventually died. During that period, it was fed fish and birds, and since Lophostaspis is primarily a genus found in marine turtles (like Caretta caretta), it may have also consumed some marine oysters from the Gulf of Mexico. So, this likely represents a pseudoparasite just passing through the turtle.
Another interesting North American aspidogastrean is Cotylogaster occidentalis. It was reported from a freshwater drum, Aplodinotus grunniens from northeastern Oklahoma, and also occurs in this host from Iowa, Louisiana, Mississippi, Tennessee, and Lake Erie, Canada. It has also been reported from freshwater mussels from Iowa, Michigan, North Dakota, and Manitoba, Canada. Since these hosts are commonly encountered in Arkansas, C. occidentalis may eventually be found in the state.
In other fishes, Cotylaspis cokeri has been reported from paddlefish (Polydon spathula) from Mississippi, and Aspidogaster decatis and A. ijimai are known from common carp, Cyprinus carpio, from Canada and Japan, respectively. All of these infections are likely the result of fish eating infected molluscs.
For additional information:
Alves, P. V., F. M. Vieira, C. P. Santos, T. Scholz, and G. L. Luque. “A Checklist of the Aspidogastrea (Platyhelminthes: Trematoda) of the World.” Zootaxa 3918 (2015): 339‒396.
Carney, J. P. “Aspidobothrean Parasites of Freshwater mussels (Bivalvia: Unionidae) from Saskatchewan-Nelson River Drainage in Manitoba, Canada and North Dakota, United States.” Comparative Parasitology 82 (2015): 9‒16.
Dickerman, E. E. “On the Life Cycle and Systematic Position of Cotylogaster occidentalis Nickerson, 1902.” Journal of Parasitology 34 (1948): 164.
Fredericksen, D. W. “Morphology and Taxonomy of Cotylogaster occidentalis (Trematoda: Aspidogastridae).” Journal of Parasitology 58 (1972): 1110‒1116.
Gibson, D. I. “Questions in Digenean Systematics and Evolution.” Parasitology 95 (1987): 429‒460.
Hoffman, G. L. Parasites of North American Freshwater Fishes. Second Edition. Ithaca: Comstock Publishing Associates, 1999.
Littlewood, D. T. J., K. Rohde, R. A. Bray, and E. A. Herniou. “Phylogeny of the Platyhelminthes and the Evolution of Parasitism.” Biological Journal of the Linnean Society 68 (1999): 257‒287.
Litvaitis, M. K., and K. Rohde. “A Molecular Test of Platyhelminth Phylogeny: Inferences from Partial 28S rDNA Sequences.” Invertebrate Biology 118 (1999): 42‒56.
McAllister, C. T., and A. Choudhury. “Cotylogaster occidentalis (Aspidogastrea: Aspidogastridae) from Freshwater Drum, Aplodinotus grunniens (Perciformes: Sciaenidae), from Northeastern Oklahoma.” Journal of the Arkansas Academy of Science 73 (2019): 143–146. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=3356&context=jaas (accessed June 5, 2021).
Park, J-K., K-H Kim, S. Kang, W. Kim, S. E. Keeseon, and D. T. J. Littlewood. “A Common Origin of Complex Life Cycles in Parasitic Flatworms: Evidence from the Complete Mitochondrial Genome of Microcotyle sebastis (Monogenea: Platyhelminthes).” BMC Evolutionary Biology 7 (2007): 11.
Rohde, Klaus. “The Aspidogastrea, Especially Multicotyle purvisi Dawes, 1941.” Advances in Parasitology 10 (1972): 77‒151.
———. “The Nervous Systems of Multicotyle purvisi Dawes, 1941 (Aspidogastrea) and Diaschistorchis multitesticularis Rohde, 1962 (Digenea). Implications for the Ecology of the Parasites.” Zeitschrift für Parasitenkunde 30 (1968): 78‒94.
———. “The Minor Groups of Parasitic Platyhelminthes. “Advances in Parasitology 33 (1994): 145‒234.
———. “Population Dynamics of Two Snail Species, Planaxis sulcatus and Cerithium moniliferum, and Their Trematode Species at Heron Island, Great Barrier Reef.” Oecologia 49 (1981): 344‒352.
———. “Sense Receptors of Multicotyle purvisi Dawes (Trematoda, Aspidobothria).” Nature 211 (1966): 820‒822.
———. “Structure and Development of Lobatostoma manteri Sp. Nov. (Trematoda, Aspidogastrea) from the Great Barrier Reef, Australia.” Parasitology 66 (1973): 63‒83.
———. “Subclass Aspidogastrea Faust & Tang, 1936.” In Keys to the Trematoda Vol. 1, edited by D. I. Gibson, A. Jones, and R. A. Bray. Wallingford, Oxon: CABI Publishing and the Natural History Museum, 2002.
Rosen, R., H. Abe, O. Adejumo, K. Ashami, L. Ballou, K. Montgomery, S. Toe, E. Berg, and L. Peng. “Mean Intensity and Prevalence of Cotylaspis insignis (Trematoda: Aspidogastridae) Infections in the Fat Mucket, Lampsilis radiata luteola (Bivalvia: Unionidae), from North Elkhorn Creek, a Tributary of the Kentucky River in Central Kentucky, U.S.A.” Comparative Parasitology 83 (2016): 1‒5.
Stromberg, P. C. “Aspidobothrean Trematodes from Ohio Mussels.” Ohio Journal of Science 70 (1970): 335‒341.
Ward, H. B., and S. H. Hopkins. “A New North American Aspidogastrid, Lophotaspis interiora.” Journal of Parasitology 18 (1931): 69‒78.
Wharton, G. W. “Studies on Lophotaspis vallei (Stossich, 1899) (Trematoda: Aspidogastridae).” Journal of Parasitology 25 (1939): 83‒86.
Wootton, D. M. “The Cotylocidium Larva of Cotylogasteroides occidentalis (Nickerson, 1902) Yamaguti 1963 (Aspidogasteridae: Aspidocotylea: Trematoda).” Proceedings of the 1st International Congress of Parasitology 1964 (1966): 547‒548.
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology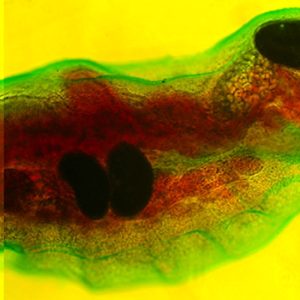 Cotylaspis insignis
Cotylaspis insignis 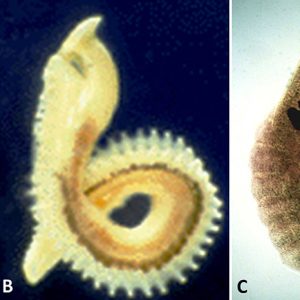 Representative Aspidogastreans
Representative Aspidogastreans 




Comments
No comments on this entry yet.