calsfoundation@cals.org
Cave Fishes
aka: Hypogean, Phreatic, Stygobitic, Subterranean, Troglomorphic, and Troglobitic Fishes
Cavefishes, found in fresh and brackish water, are adapted to living in caves and other subterranean habitats. There are more than 200 species of obligatory cavefishes found on all continents, except for Antarctica. New cavefish species continue to be described annually, and there are certainly undescribed species yet to be discovered. In terms of relative numbers of species on various continents, by far the greatest diversity is in Asia with more than 120 species, followed by South America with more than thirty species, and about thirty species in North America. In comparison, nine species are known from Africa, five from Oceania, and only a single species from Europe. China possesses the greatest diversity with more than eighty species, followed by Brazil with more than twenty species, and India, Mexico, Thailand, and the United States of America each have nine to fourteen species; as of 2021, no other country has more than five cavefish species. Arkansas hosts the Ozark cavefish and southern cavefish.
Although widespread as a group, many cavefishes have very small ranges, are generally endemic (often restricted to a single cave or cave system), and have populations that are seriously threatened or endangered. The majority occur in tropical or subtropical waters and are strongly associated with karst regions, which commonly produce underground sinkholes and subterranean rivers. Many species are restricted to caves with underground lakes, pools, or rivers; however, some are found in aquifers and may only be detected when artificial wells are drilled, pumped, or otherwise opened up. Most cavefishes occur in regions with low (essentially static) or moderate water current, but there are also species in sites with very strong current, such as the waterfall climbing cavefish (Cryptotora thamicola), a species of troglobitic hillstream loach endemic to Thailand. Cryptotora thamicola is known for its fins, which can grasp onto terrain, and for its ability to climb. Although most cavefishes are found in freshwater habitat, a few species live in anchialine caves, and several of these tolerate various salinities, most notably the cave-dwelling viviparous Mexican blind brotula (Typhliasina pearsei), Luciogobius gobies, Milyeringa sleeper gobies, and the blind cave eel (Ophisternon candidum).
Obligate cavefishes are considered extremophile members within a wide range of families, and many are only very distantly related and do not form a monophyletic group, showing that adaptations to a life in caves have evolved numerous times. Familial examples include Amblycipitidae (torrent catfishes), Amblyopsidae (U.S. cavefishes), Astroblepidae (naked sucker-mouth catfishes), Balitoridae (hillstream loaches), Butidae (butid gobies), Bythitidae (brotulas), Callichthyidae (armored catfishes), Characidae (characids), Clariidae (airbreathing catfishes), Cobitidae (true loaches), Cottidae (true sculpins), Cyprinidae (carps and allies), Eleotridae (sleeper gobies), Gobiidae (gobies), Heptapteridae (heptapterid catfishes), Ictaluridae (ictalurid catfishes), Kryptoglanidae (kryptoglanid catfish), Loricariidae (loricariid catfishes), Milyeringidae (blind cave gobies), Nemacheilidae (stone loaches), Phreatobiidae (phreatobiid catfishes), Poeciliidae (live-bearers), Sternopygidae (glass knifefishes), Synbranchidae (swamp eels), and Trichomycteridae (pencil catfishes).
Cavefishes have a unique suite of adaptations to survive in a unique niche with few resources, such as food. Typical adaptations of troglobitic (cave-obligate) species include reduced eyes, lack of body pigmentation, low metabolic rates, and subsistence under thermal constancy. Because troglobitic cavefishes occur in total or near darkness, the presence of pigmentation and eyes are useless, or could be an actual disadvantage because of their energy requirements. Also, the loss of eyes can be complete or only partial. A few species possess small or incomplete eyes, and eyes can actually be present in the earliest life (larval) stages but degenerate when maturing to the adult stage. Interestingly, so-called “blind” cavefishes may still be able to see. For example, troglomorphic forms of juvenile Mexican tetras (Astyanax mexicanus) are eyeless and found widely distributed from New Mexico and Texas, through Mexico, and farther south into Guatemala. They are able to sense light via certain cells in their pineal gland (pineal eye). Others, like Congo blind barbs (Caecobarbus geertsi) from the lower Congo River basin in the Democratic Republic of the Congo are photophobic, in spite of only having retinas and optical nerves that are rudimentary and located deep inside the head, and completely lacking a lens.
Without the sense of sight, other senses are utilized, and these may actually be enhanced. Examples include the lateral line for sensing vibrations, chemoreception (via smell and taste buds), and mouth suction to sense nearby obstacles (comparable to echolocation). Despite lacking eyes, some cavefishes respond to light and move away from it (termed scotophilia). Other examples of morphological adaptations are larger fins for more energy-efficient swimming, and a loss of scales and a swim bladder. The level of specialized adaptations in a cavefish is generally considered to be directly correlated to the amount of time it has been restricted to the underground habitat. For example, species that recently arrived show fewer adaptations, and species with the largest number of adaptations are likely the ones that have been restricted to the habitat for the longest time.
Cavefishes are typically small, with most species being less than 10 cm (4 in.) long with very few surpassing 20 cm (8 in.) in length. However, the longest known cavefish, the blind cave eel (O. candidum), endemic to subterranean waters in the Cape Range, Australia, reaches a length of up to 40 cm (16 in.).
The restricted food resources in caves likely prevent larger cavefish species from existing and also means that cavefishes, in general, are opportunistic feeders, taking whatever is readily available. Cavefish are often apex predators, feeding on smaller cave-adapted invertebrates—such as copepods, isopods, amphipods, and small crayfish—or are detritivores. As most cavefishes lack natural predators, however, larger cavefish may be cannibalistic on smaller individuals. In addition, cave-adapted blind crayfishes, crabs, giant water bugs, and spiders have been recorded feeding on a few species of cavefishes. Some species are able to survive long periods of starvation, and, as such, these species can build up unusually large, fat bodies by “binge eating” in periods where food is more readily available.
In general, cavefishes are slow growing and are deliberate breeders. Reproductive behaviors among cavefishes vary extensively, and there are both species that are egg-layers and species that give birth to live young. Uniquely among cavefishes, the genus Amblyopsis brood their eggs for up to four or five months in the branchial gill chambers, similar to that of mouth-brooding fishes, common in cichlids.
Being in subterranean environs, many sites and associated microhabitats where cavefish live have not been thoroughly surveyed. For example, the first cavefish in Europe, a Barbatula stone loach, was only discovered in 2015 in southern Germany near the Swabian Alb. The fish was found in the 250 km2 (96.5 mi2) underground karst water system embedded in the limestone formation of the White Jura known as the “Danube-Aach system.” The oldest known description of an obligate cavefish, the Hyaline fish (Sinocyclocheilus hyalinus), a threatened species found only in Yunnan, China, was mentioned in 1540 in the travel notes of Yingjing Xie, a local governor of Guangxi.
Although cavefishes as a group are found throughout large parts of the world, many cavefish species have restricted ranges and are seriously threatened. The critically endangered golden cave catfish (Clarias cavernicola), with an estimated population of less than 400 individuals, is found only in the Aigamas Cave in Namibia (Otjozondjupa region). In addition, the Haditha cavefish (Caecocypris basimi), a species of cyprinid fish endemic to western Iraq, only occurs in aquifers near Haditha (Al Anbar Governorate). The species is classified as critically endangered and possibly extinct by the International Union for Conservation of Nature (IUCN), as there have been no specimens recorded since 1983 despite a comprehensive survey in 2012.
Living in very constant thermal environments, cavefishes are more likely to be vulnerable to changes in the aquatic environment (e.g., changes in water temperature or oxygen concentration) than fishes in epigean habitats, which naturally experience greater environmental variations. The main threats to cavefishes are typically changes in the water level (mainly through deliberate water extraction or drought conditions), habitat degradation and pollution, and human intrusion and destruction of caves. However, in some cases, introduction of exotic species and unauthorized collection of specimens for the aquarium trade may also pose a threat.
Some interesting North American cavefishes include the Hoosier cavefish (Amblyopsis hoosieri), which was described in 2014 from southern Indiana and represented the first new species of amblyopsid cavefish to be discovered in forty years in the United States. It is distributed among sixty-eight caves and six springs throughout Indiana, between the Ohio River and the East Fork of the White River. The Alabama cavefish (Speoplatyrhinus poulsoni) is a critically endangered species of amblyopsid cavefish found only in northwestern Alabama within underground pools of Key Cave. It was originally discovered by crayfish biologist John E. Cooper (1929‒2015) underneath a colony of gray bats (Myotis grisescens) in 1967 and formally described in 1974. It is thought to be the rarest species of cavefish in the United States and one of the scarcest of all freshwater fish. It exists in a fragile ecosystem based on nutrient-rich gray bat guano. The Northern cavefish (Amblyopsis spelaea) is found in caves of the Pennyroyal and Mitchell plateaus, from the Mammoth Cave area, central Kentucky, north to the Ohio River. Northern Cavefish inhabit subterranean waters or limestone caves that have consolidated mud-rock substrates in shoals or in silt-sand substrates in pools; however, it is more often found in caves with uniform silt-sand substrates. They are very vulnerable to contamination or habitat changes that potentially occur in the cave stream. The toothless blindcat (Trogloglanis pattersoni) and widemouth blindcat (Satan eurystomus) are both from the abysmal San Antonio Pool of the Edwards Aquifer directly under the city of San Antonio, Texas. They were originally collected by hand from water flowing from artesian (natural pressure) wells and are the only known stygobitic (obligate troglobitic) catfishes in the United States. These fish come only from a few wells in the Edwards Aquifer that are more than 300 m (984 ft.) deep. Their true population size and trends are unknown, and both are vulnerable to pollution and depletion of the aquifer. The spring cavefish (Forbesichthys agassizii) is a troglophilic species (occurring in but not restricted to caves) with a dusky-pigmented body and poorly developed (but visible) eyes. It occurs in the mouths of caves and springs from Highland Rim area of Tennessee River drainage, middle and lower Cumberland River drainage, upper Barren-Green system of Kentucky, and Ohio and Mississippi river tributaries near their junction, including one population west of the Mississippi in Missouri. This species is now considered one of two species: F. agassizii or F. papilliferus.
In Arkansas, the Ozark cavefish (Troglichthys [formerly Amblyopsis] rosae) was described by the German American ichthyologist Carl H. Eigenmann (1863‒1927) in 1898. To date, T. rosae exists in forty-one caves and wells in Arkansas, Missouri, and Oklahoma. Ozark cavefish are found in a small range within the Springfield Plateau of the Ozark Mountains to include an area drained by the Osage River (Missouri River basin) to the north, the Neosho River (Arkansas River basin) on the west, and the White River on the east and the south. Recent molecular data indicates that the Ozark cavefish comprises four genetically distinct clades in the following drainages: (1) White River, Missouri; (2) Neosho River, Missouri; (3) Neosho River, Oklahoma; and (4) Illinois River, Arkansas. Molecular data suggests these clades may warrant recognition as subspecies. The largest known population of T. rosae is at Cave Springs (Benton County) and has apparently recouped in recent years from past extensive over-collecting. The true population size is unknown. Most are of uncertain trend, although some populations are apparently stable while others are seemingly declining.
Morphological features of T. rosae include absent pelvic fins and rounded caudal fin. The pectoral fins are elongated, anal and urogenital openings are jugular (far in front of anal fin toward the throat), cycloid scales are minute and embedded, and sensory papillae (4 to 6 rows on the anal fin) are well developed. They have a broadly elongated flattened head, with a prominent lower jaw and rudimentary eyes hidden under the skin, and are without pigment. Adults average about 50 mm (2 in.) long but may reach nearly 70 mm (3 in.) in length.
Individuals of this species are found mostly in the dark zone of caves having large permanent streams but permanently in small cave streams with rubble or chert substrate, in pools over sand and silt bottom, or in karst fensters or wells. Suitable caves inhabited by the Ozark cavefish all have a relatively significant source of organic nutrients, such as bat guano or blown leaf litter. These fish can tolerate extremely low oxygen contents in water and have a reduced metabolism, spending most of their time stationary in their habitat. They feed mostly on small crustaceans (amphipods, copepods, and isopods), small crayfish, and larval salamanders, but cases of cannibalism have been reported.
In terms of reproduction, it is thought that their eggs, numbering up to seventy, are carried in the gill chamber of the female until they hatch; the young remain there for a period of four to five months until the yolk is absorbed. The species is slow growing, with a relatively long life span (about ten years), with maturity taking about four years.
This species has been ranked as critically imperiled (S1) in Arkansas by NatureServe and classified as threatened by the U.S. Fish and Wildlife Service. Some threats to overall survival include potential negative effects to both water quality and quantity that is ultimately associated with expansion and other land use development activities, a lack of knowledge regarding the species natural history and ecology, potential indirect effects to the species by white nose syndrome caused by the fungus Pseudogymnoascus destructans harming bat populations, trespass and vandalism, and mortality/habitat damage resulting from cave entry by humans. In addition, several populations have apparently been extirpated as a result of stocking of trout, filling in of cave/sinkhole entrances, contaminant spills, or flooding by reservoirs.
The southern cavefish (Typhlichthys eigenmanni [formerly subterraneus]) is the other species of troglobitic cavefish found in Arkansas and was described in 1859 by French biologist Charles F. Girard (1822‒1895). It is unusual among cavefishes, as it has the largest known distribution of any subterranean fish in the world, spanning more than 5° of latitude and over 140,000 km2. The current discontinuous distribution includes caves and karst ranging on both sides of the Mississippi River, with two main populations as follows: (1) east in northwest Alabama, northwest Georgia, central Tennessee, and Kentucky, and (2) west in central and southeastern Missouri and northeastern Arkansas. In Arkansas, Ty. eigenmanni has been reported from the Ozark Highlands at Richardson (Martin) Cave (Fulton County), Alexander (Castle) Cave/Clark Spring (Stone County), Ennis Cave (Stone County) and Norfolk Lake (North Fork River, Baxter County).
This cavefish usually inhabits water bodies at considerable depths averaging about 200 m (656 ft.) below surface land. They are found mostly in flowing waters and appear to be attracted to point sources of water efflux. This species also displays a strong preference for large substrate sizes. For example, they may use the larger spaces in between the pebbles for protection from both predators and strong currents which, in turn, may facilitate the fish dispersal. Like Tr. rosae, southern cavefish tend to rest motionless on the bottom for extended periods of time. Estimations of population sizes based on sightings at a single cave usually yield fewer than 150 individuals.
This species, which is larger than Tr. rosae, can grow to standard lengths of 90 mm (3.5 in.). It possesses a large, broad head with flattened body that exhibits simple eyes hidden under the skin; scales are minute and embedded. The caudal fin has from zero to two rows of sensory papillae (one on the upper half and the other on the lower half) and a vertical basal row. Fin ray counts are as follows: 7 to 10 (7 to 10) dorsal soft rays, 7 to 9 anal soft rays, 9 to 12 pectoral fin rays, no pelvic fins, and 10 to 15 branched caudal rays. The anus is well in front of the anal fin in adults. The body is depigmented or slightly pink, with some non-functional pigment cells. The southern cavefish has a swim bladder and numerous sensory cells (neuromasts) with cylindrical bulbous cupulae lying in a row and alternating left and right of center in the body. They also have many small unicuspidal teeth.
Members of this species live for a long time (possibly even for decades) and grow slowly. Breeding probably occurs between April and May in association with rising water levels. Up to fifty percent of the adult females of a population may breed in any given year. They become reproductively active at twenty-two to twenty-four months of age. Fecundity is reduced, perhaps to fewer than fifty eggs (ova) per female. It takes about two months for free-swimming young to develop from the fecundated (zygote) stage. Females display parental care.
Members of this species feed mostly on copepods (60 to 80 percent of their diet), but remains of other organisms have been found in their digestive system, such as amphipods, isopods, decapods, ostracods, cladocerans, non-annelid worms, aquatic insects (both adults and larvae), algae, and debris.
In terms of threats, they are similar to those of Tr. rosae but also include percolation of agricultural chemicals or other contaminants and sedimentation in its underground subterranean environment. In addition, populations in some sites are possibly declining, but trends are poorly known because most of the habitat is inaccessible. Populations in Arkansas are ranked S1 (critically imperiled) by NatureServe.
A study conducted on several former populations of Ty. subterraneus and published in 2012 advocated restoration of Ty. eigenmanni for Ozark Highland populations of Typhlichthys. The authors stated that the discovery of significant genetic variation across the range of Ty. subterraneus warranted further detailed investigations of species delimitation in these cavefish. This conclusion was based on biogeographical and phylogenetic evidence, and further work will likely result in recognition of additional species both east and west of Mississippi River.
In terms of endoparasites, a tapeworm has been reported from A. spelaea, but little is known about those from other North American cavefishes, and nothing has been published on parasites of any cavefish from Arkansas. However, a new nematode species, Rhabdochona longleyi, was described from the intestine of T. pattersoni and S. eurystomus from Texas.
For additional information:
Adams, Ginny L., Brooks M. Burr, J. L. Day, and D. E. Starkey. “Cottus specus, a New Troglomorphic Species of Sculpin (Cottidae) from Southeastern Missouri.” Zootaxa 3609 (2013): 484–494.
Behrmann-Godel, Jasminca, Arne W. Nolte, Joachim Kreiselmaier, Roland Berka, and Jörg Freyhof. “The First European Cave Fish.” Current Biology 27 (2017): R257‒R258.
Brown, Arthur V., and C. Stan Todd. “Status Review of the Threatened Ozark Cavefish (Amblyopsis rosae).” Proceedings of the Arkansas Academy of Science 41 (1987): 99–100. Online at https://scholarworks.uark.edu/jaas/vol41/iss1/28/ (accessed September 16, 2021).
Chakrabarty, Prosanta, Jacques A Prejean, and Matthew L. Niemiller. “The Hoosier Cavefish, a New and Endangered Species (Amblyopsidae, Amblyopsis) from the Caves of Southern Indiana.” ZooKeys 412 (2014): 41–57.
Cooper, John E., and R. A. Kuehne. “Speoplatyrhinus poulsoni, a New Genus and Species of Subterranean Fish from Alabama.” Copeia 1974 (1974): 486‒493.
Dunivan, James D., C. Renn Tumlinson, and V. Rick McDaniel. “Cave Fauna of Arkansas: Further Records.” Proceedings of the Arkansas Academy of Science 36 (1982): 87‒88. Online at https://scholarworks.uark.edu/jaas/vol36/iss1/28/ (accessed September 16, 2021).
Espinasa, L., and W. R. Jeffery. “A Troglomorphic Sculpin (Pisces: Cottidae) Population: Geography, Morphology and Conservation Status.” Journal of Cave and Karst Studies 65 (2003): 93–100.
Etnier, David A., and Wayne C. Starnes. The Fishes of Tennessee. Knoxville: University of Tennessee Press, 1993.
Green, S., and Aldemaro Romero. “Responses to Light in Two Blind Cave Fishes (Amblyopsis spelaea and Typhlichthys subterraneus) (Pisces: Amblyopsidae).” Environmental Biology of Fishes 50 (1997): 167‒174.
Hoffman, Glenn L. Parasites of North American Freshwater Fishes. 2nd ed. Berkeley: University of California Press, 2009.
Jones, S. R., and C. A. Taber. “A Range Revision for Western Populations of the Southern Cavefish Typhlichthys subterraneus (Amblyopsidae).” American Midland Naturalist 113 (1985): 413‒415.
Keith, J. H. “Distribution of Northern Cavefish, Amblyopsis spelaea DeKay, in Indiana and Kentucky and Recommendations for its Protection.” Natural Areas Journal 8 (1988): 69‒79.
Kuhajda, Bernard R., and Richard L. Mayden. “Status of the Federally Endangered Alabama Cavefish, Speoplatyrhinus poulsoni (Amblyopsidae), in Key Cave and Surrounding Caves, Alabama.” Environmental Biology of Fishes 62 (2001): 215‒222.
Langecker, Thomas G., and Glenn Longley. “Morphological Adaptations of the Texas Blind Catfishes Trogloglanis pattersoni and Satan eurystomus (Siluriformes: Ictaluridae) to Their Underground Environment.” Copeia 1993 (1993): 976‒986.
Means, M. L., and J. E. Johnson. “Population Dynamics and Movement of Ozark Cavefish in Logan Cave National Wildlife Refuge, Arkansas.” Southwestern Naturalist 40 (1995): 308‒313.
Mettee, M. F., P. E. O’Neil, and J. M. Pierson. Fishes of Alabama and the Mobile Basin. Birmingham: Oxmoor House, 1996.
Miller, Rudolph J., and Henry W. Robison. Fishes of Oklahoma. Norman: University of Oklahoma Press, 2004.
NatureServe. https://explorer.natureserve.org/ (accessed September 16, 2021).
Noltie, Douglas B., and Carol M. Wicks. “How Hydrogeology Has Shaped the Ecology of Missouri’s Ozark Cavefish, Amblyopsis rosae, and Southern Cavefish, Typhlichthys subterraneus: Insights on the Sightless from Understanding the Underground.” Environmental Biology of Fishes 62 (2001): 171–194.
Page, Larry M., and Brooks M. Burr. Peterson Field Guide to Freshwater Fishes of North America North of Mexico. 2nd ed. Boston: Houghton Mifflin Harcourt, 2011.
Paige, Kenneth, C. Renn Tumlison, and V. Rick McDaniel. “A Second Record of Typhlichthys subterraneus (Pisces: Amblyopsidae) from Arkansas.” Southwestern Naturalist 26 (1981): 67‒92.
Pflieger, William L. The Fishes of Missouri. Jefferson City: Missouri Department of Conservation, 1997.
Poulson, Thomas L. “Cave Adaptation in Ambylopsid Fishes.” American Midland Naturalist 70 (1963): 257‒290.
Poulson, Thomas L., and W. B. White. “The Cave Environment.” Science 165 (1969): 971–981.
Proudlove, G. S. “The Conservation of Hypogean Fishes.” Environmental Biology of Fishes 62 (2001): 201‒213.
Robison, Henry W., and Thomas M. Buchanan. Fishes of Arkansas. 2nd ed. Fayetteville: University of Arkansas Press, 2020.
Romero, Aldemaro. Cave Biology: Life in Darkness. New York: Cambridge University Press, 2009.
———. “Threatened Fishes of the World: Amblyopsis rosae (Eigenmann, 1842) (Amblyopsidae).” Environmental Biology of Fishes 52 (1998): 434.
Romero, Aldemaro, M. S. Connor, and G. L. Vaughan. “Population Status of the Southern Crayfish, Typhlichthys subterraneus in Arkansas.” Journal of the Arkansas Academy of Science 64 (2010): 106–110. Online at https://scholarworks.uark.edu/jaas/vol64/iss1/22/ (accessed September 16, 2021).
Romero, S., and S. M. Green. “The End of Regressive Evolution: Examining and Interpreting the Evidence from Cave Fishes.” Journal of Fish Biology 67 (2005): 3‒32.
Willis, Lawrence D., and Arthur V. Brown. “Distribution and Habitat Requirements of the Ozark Cavefish, Amblyopsis rosae.” American Midland Naturalist 114 (1985): 311‒317.
Woods, Loren P., and Robert F. Inger. “The Cave, Spring, and Swamp Fishes of the Family Amblyopsidae of Central and Eastern United States.” American Midland Naturalist 58 (1957): 232–256.
Chris T. McAllister
Eastern Oklahoma State College

 Cave-adapted Fishes
Cave-adapted Fishes 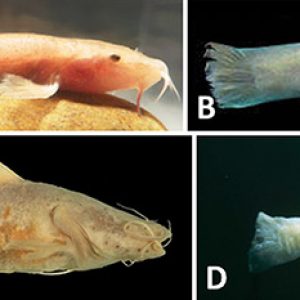 Cave-adapted Fishes
Cave-adapted Fishes 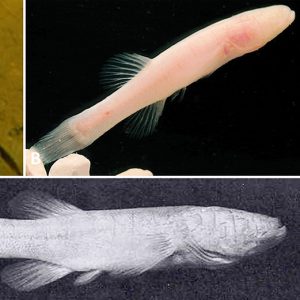 North American Cave-adapted Fishes
North American Cave-adapted Fishes  Ozark Cavefish
Ozark Cavefish  Ozark Cavefish
Ozark Cavefish  Southern Cavefish
Southern Cavefish 



Comments
No comments on this entry yet.