calsfoundation@cals.org
Cladocerans
aka: Water Fleas
Water fleas (cladocerans) belong to the Phylum Arthropoda, Subphylum Crustacea, Class Branchiopoda, and Order Cladocera. Over 700 species and more than 100 genera have been recognized, but many additional species are surely undescribed. The genus Daphnia alone contains around 150 species. The order forms a monophyletic group, which is divided into four suborders and eleven families as follows: Anomopoda (five families), Ctenopoda (two families), Haplopoda (one family), and Onychopoda (three families). Although a complete survey of the cladocerans of Arkansas has not been given, about twenty species/taxa within five families have been reported.
Cladocerans first appeared in the Permian. Until recently, the evolutionary history of cladocerans has been obscured by a mixture of erroneous fossil identifications and assumptions. However, knowledge of their fossil record and understanding of freshwater paleo-ecosystems and their communities have made important gains over the last several decades, but further analysis of cladoceran paleontological data is still needed. The Cladocera exhibit a striking morphological stasis over millions of years, yet they have overcome significant ecological shifts and continue to show remarkable plasticity, which has been the key to their survival and success in the present day.
Cladocerans are among the most successful invertebrates in freshwater ecosystems (including inland water bodies), whereas about eight species have also adapted to a living in marine environments, and several anostracans live in hypersaline lakes. Except for the genus Penilia, the marine species all belong to the family Podonidae. In all these waters, most are benthic crawlers or burrowers, while others are planktonic. They are key primary consumers and an important component of the zooplankton and of prime importance in the global aquatic food chain. Those in the genus Moina have demonstrated the ability to survive in waters containing low oxygen levels as well as high salinities and other impurities, including salt pans and water than has undergone eutrophication.
Anatomically, cladocerans possess a head that is angled downward and may be separated from the rest of the body by a notch or “cervical sinus.” Located on the organism’s midline (in all but two genera) is a single median black compound eye, and a single ocellus is also frequently present. The body is not obviously segmented and bears a folded (only dorsally) carapace that covers the thorax and abdomen (trunk). In addition, the head includes two pairs of antennae as follows: the first antennae are small, unsegmented appendages with olfactory setae, while the second antennae are large, segmented, and branched, with powerful muscles used for swimming by most species. Interestingly, the pattern of the setae on the second antennae is taxonomically useful for identification. The part of the head that projects in front of the first antennae is known as the rostrum or “beak.” The mouthparts are small and consist of an unpaired labrum, a pair of mandibles, a pair of maxillae, and an unpaired labium. The thorax bears five or six pairs of lobed, leaf-like appendages, each with numerous hairs or setae. Most cladocerans are 0.2–6.0 mm (0.01–0.24 in.) in length. The exception is Leptodora, which can be up to 18 mm (0.71 in.) long.
Most benthic cladocerans feed on organic material by scraping from sediment particles or other objects, whereas the planktonic species are suspension feeders. There are also some (e.g., Bythotrephes, Leptodora) that are predators on other cladocerans. One genus of cladoceran, Anchistropus, is an epibiont on Hydra.
Many cladocerans exhibit cyclical parthenogenesis, by which asexual reproduction is occasionally augmented by sexual reproduction. This specialized form of reproduction produces resting eggs that allow certain species to survive harsh conditions (periods of overcrowding, adverse temperatures, or scarce food) and disperse into distant habitats. However, when conditions are more favorable, reproduction occurs by parthenogenesis for several generations, producing only female clones. In addition, as the conditions deteriorate, males are produced and sexual reproduction occurs. This results in the production of long-lasting dormant or “ephippial” eggs. All ephippia are resistant to adverse environmental conditions and can remain dormant in sediment for long periods of time, delaying hatching until optimal conditions are present. These eggs can then be transported overland by wind action, allowing many species to have very wide, even cosmopolitan, geographic distributions. A phenomenon called cyclomorphism occurs in many planktonic forms that undergo seasonal changes in body form through succeeding generations of parthenogenetically produced individuals.
Several cladocerans have become invasive species, including the spiny water flea (Bythotrephes longimanus), fishhook water flea (Cercopagis pengoi), and Daphnia lumholtzi. The spiny water flea and fishhook water flea are native to fresh waters of Asia and Northern Europe but have been accidentally introduced and widely distributed since the 1980s in the Great Lakes of North America. Daphnia lumholtzi has been introduced into North America, including Arkansas, in the watersheds of Bayou Macon, Beaver Reservoir, Bayou Bodcaw, Dardanelle Reservoir, Lower Arkansas, Lake Maumelle, Lower Ouachita/Bayou De Loutre, Lower Saline, Lower St. Francis, Lower White/Bayou Des Arc, and Upper Ouachita. The impacts of this invader are not yet fully understood, although it is known to compete with native Daphnia spp. for food.
In Arkansas, although a complete survey of the cladocerans of the state has not been given, the following species/taxa within five families have been reported, including: Bosmina longirostris, B. coregoni, Daphnia parvula, D. similis, D. magna, D. middendorffiana, D. ambigua, D. galeata, D. rosea, Ceriodaphnia lacustris, C. quadrangula, C. reticulata, C. pulchella, Moina micrura, M. brachiata, Holopedium amazonicum, H. gibberum, Diaphanosoma brachyurum, D. leuchtenbergianum, Sida sp., and Chydorus sphaericus. A comprehensive survey is sorely needed.
For additional information:
Frisch, D., and Lawrence J. Weider. “Seasonal Shifts in Genotype Frequencies in the Invasive Cladoceran Daphnia lumholtzi in Lake Texoma, U.S.A.” Freshwater Biology 55 (2010): 1327‒1336.
Havel, J. E. “Environmental Limits to a Rapidly Spreading Exotic Cladoceran.” Ecoscience 12 (2005): 376‒385.
Hynes, H. B. N. The Ecology of Running Waters. Ontario, Canada: University of Toronto Press and University of Waterloo, 1970.
Jackson, D. C., and Eugene H. Schmitz. “Zooplankton Abundances in Vegetated and Non-Vegetated Areas: Implications for Fisheries Management.” Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 41 (1987): 214‒220.
Palko, Tom N. “A Preliminary Study of Zooplankton over a Six Month Period on Lake Dardanelle.” Proceedings of the Arkansas Academy of Science 24 (1970): 55‒61. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2991&context=jaas (accessed January 10, 2020).
Rickett, John D., and Robert L. Watson. “First Record of Leptodora kindti in Dardanelle Reservoir and Status of Other Recent Additions to Dardanelle Fauna.” Proceedings of the Arkansas Academy of Science 48 (1994): 261‒263. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1970&context=jaas (accessed January 10, 2020).
Roseberg, Ralph B., and Mark Karnes. “The Influence of DeGray Reservoir on Zooplankton Populations in the Caddo and Ouachita Rivers.” Proceedings of the Arkansas Academy of Science 38 (1984): 96‒97. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2510&context=jaas (accessed January 10, 2020).
Roseberg, Ralph B., and Thomas Moen. “New Record of Leptodora kindtii (Focke) (Crustacea: Cladocera) in Arkansas.” Southwestern Naturalist 26 (1981): 74–75.
Rubbo, Michael J., and Joseph M. Kiesecker. “Leaf Litter Composition and Community Structure: Translating Regional Species Changes into Local Dynamics.” Ecology 85 (2004): 2519–2525.
Schram, Mark D., Arthur V. Brown, and D. C. Jackson. “Diel and Seasonal Drift of Zooplankton in a Headwater Stream.” American Midland Naturalist 123 (1990): 135‒143.
Short, Edgar D., and Eugene H. Schmitz. An Evaluation of the Effects of Dedging within the Arkansas River Navigation System. Volume III. Effects Upon the Zooplankton Associations. Final Report to the United States Corp of Engineers Contract No. DACWO 1976 3-74-C-0-146. Arkansas Water Resources Center, University of Arkansas, Fayetteville, 1976.
Smith, Stephen B., and Thomas E. Moen. “Zooplankton Population Structure in Three Reservoirs near the Ouachita Mountain-Gulf Coastal Plain Interface.” Proceedings of the Arkansas Academy of Science 37 (1983): 99‒100. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2552&context=jaas (accessed January 10, 2020).
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology Cladocerans
Cladocerans 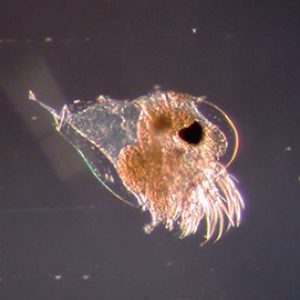 Cladocerans
Cladocerans 



Comments
No comments on this entry yet.