calsfoundation@cals.org
Ticks
Ticks belong to the phylum Arthropoda, class Arachnida, subclass Acari, suborder Parasitiformes, and order Ixodida (Metastigmata), which includes almost 900 recognized species. There are three families: Ixodidae, or the “hard” ticks (approximately 700 species); Argasidae, or the “soft” ticks (approximately 200 species); and Nuttalliellidae, containing only a single species, Nuttalliella namaqua, a tick found only in southern Africa. In Arkansas, nine genera and a total of nineteen species (three argasids and sixteen ixodids) are known. Another species, Rhipicephalus (Boophilus) annulatus, has been extirpated from Arkansas.
Ticks are a highly specialized group of obligate, bloodsucking, nonpermanent ectoparasitic arthropods of vertebrates (mostly on reptiles, birds, and mammals) and are distributed throughout the world. In addition to being irritating to hosts and causing blood loss, damage to the skin, and anorexia leading to reduction in growth, ticks are important to human and veterinary medicine as vectors of bacterial, protozoal, rickettsial, spirochaetal, and viral disease agents of humans, domestic stock, and other mammals. Ticks are large mites, and rDNA analysis suggests that the Ixodidae are monophyletic (that is, consisting of a single ancestral species and its descendants), but that the Argasidae may be paraphyletic. Recent data suggest that Nuttalliella namaqua is the basal group from which both the Argasidae and Ixodidae evolved.
Fossilized ticks known from the Cretaceous period are often found in amber (fossilized tree resin). They are thought to have originated sixty-five to 146 million years ago during the Cretaceous Period, with most of their evolution and dispersal occurring five to sixty-five million years ago during the Tertiary period. The oldest example of a fossilized tick is an argasid species from a bird from the Cretaceous found in New Jersey amber.
One of the most famous tick biologists was Harry Hoogstral (1917–1986). He was described as “the greatest authority on ticks and tickborne diseases who ever lived.” Others include George Henry Falkiner Nuttall (1862–1937), who published on ticks and tickborne diseases; Robert A. Cooley (1873–1968), who is known for his vast collection of African ticks and many publications on ticks of the world; and Wilhelm “Willy” Burgdorfer (1925–2014), who is known for his discovery of the Lyme disease spirochete and its relation to tick vectors in addition to his research activities on ticks carried out at the Rocky Mountain Laboratory (RML) in Hamilton, Montana. Two famous tick biologists of the modern era include James H. Oliver Jr. (born 1931), who provided invaluable contributions in the study of tickborne spirochetes, and James E. Keirans (born 1935), former curator and tick biologist of the U.S. National Tick Collection (USNTC). In Arkansas, tick researcher J. L. “Jay” Lancaster Jr. (1923–2016) was known for his work on the Lone Star tick and rising human fatalities from Rocky Mountain spotted fever. He also published extensively on Arkansas ticks. He was professor of medical and veterinary entomology at the University of Arkansas (UA) in Fayetteville (Washington County) until his retirement in 1993 after forty-three years of service.
Morphologically, ticks possess a hypostome (a portion of the mouthparts) with teeth, and it is usually exposed anteriorly. A depression of the first tarsi called Haller’s organ is present, and a pair of spiracles lie near the coxae of a fourth pair of legs in nymphs and adults. Haller’s organ functions as an olfactory and humidity receptor. In hard ticks, the dorsal idiosomal (body) surface is covered by a sclerite termed a scutum; in adult females, the scutum does not cover the entire dorsal surface so that engorging may easily occur. Some species possess eyes on the scutum, whereas other species have no eyes.
Hard ticks of the family Ixodidae are mostly dioecious (that is, non-hermaphroditic, with separate male and female organisms), and females usually require a blood meal prior to producing eggs. Copulation mostly takes place on the host; females then usually drop off and lay the eggs in soil or leaf litter. A six-legged larval stage hatches from the egg and requires a blood meal to molt. An eight-legged nymph follows, which requires a blood meal to molt into the adult tick. Both ixodid and argasid ticks have four stages in their life cycle: egg, followed by three active parasitic stages—larva, nymph, and adult (males and females). Several species of argasids have more than one nymphal instar.
Hard ticks seek out hosts by a fascinating behavior called “questing.” Questing ticks crawl up the stems of grass or perch on the edges of leaves on the ground in a posture with the front legs extended, especially in response to a host traveling by. These ticks climb onto a potential host that brushes against their extended front legs.
Hard ticks are most commonly collected for scientific research by the use of “flags” or “drags,” which are made from one-square-meter pieces of rough-textured fabric such as fleece or flannel attached to a rod handle. The flags are slowly dragged across the surface of vegetation to collect questing ticks. Soft ticks can be collected via dry ice (solid carbon dioxide) traps. Blocks of dry ice emit large amounts of the gas, which serves as a host-seeking stimulant. Traps are set in and around nesting areas of animal hosts.
Ticks possess different host-seeking strategies depending on how many hosts they require: 1) one-host ticks spend all their life-cycle stages on the same host; 2) two-host ticks generally spend their larval and nymphal stages on one host, but the nymph drops off to molt and the adult seeks another (second) host; and 3) three-host ticks represent the vast majority of ixodid species. All stages of three-host ticks drop off the host prior to molting, and the next stage needs to seek out a new host. Although some species are host specific, many are generalists and can survive for months and even years without taking a blood meal.
There are eleven genera and about 200 described species of Argasidae in the world. One genus (Argas) is mostly found as ectoparasites of bats and birds. Their body is obviously textured (wrinkled). One species, the fowl tick (Argas persicus), infests many types of birds, including chickens and turkeys. Another, A. cooleyi, is found on cliff swallows and in their nests. Another genus (Ornithodoros) includes about 100 species, with many of them feeding on bats. It may be the most important genus with respect to pathogen transmission. The body is minutely wrinkled, and like Argas spp., most are nocturnal feeders. Examples include O. capensis on marine birds and O. hermsi on rodents (sometimes humans) in the western United States.
The three species of soft ticks in Arkansas are Carios (Ornithodoros) kelleyi on pigeons, Ornithodoros concancensis on eastern phoebes, and Otobius megnini (spinose ear tick) on cattle. The latter species has spiny nymphs, and the larvae and nymphs prefer to feed just inside the ear of cattle and other ungulates; adults do not feed. Ranchers have used acaricide-impregnated ear tags for controlling O. megnini on Arkansas livestock. There do not appear to be any currently established foci of this tick in the state, but humans visiting endemic areas or livestock being imported could be infested. A similar species, Otobius lagophilus, feeds on faces of rabbits in western North America, but not Arkansas. In soft ticks, the capitulum is subterminal in nymphs and adults, there is no scutum or festoons, and the body is leathery. Their mouthparts are recessed in an antero-ventral cavity and cannot be seen from above. Male and female soft ticks are morphologically identical except for minor differences in their genital apertures. There are typically five nymphal stages, and most species feed often. Unlike hard ticks, they do not engorge and feed quite rapidly (in a matter of minutes), while larvae usually attach and feed for days.
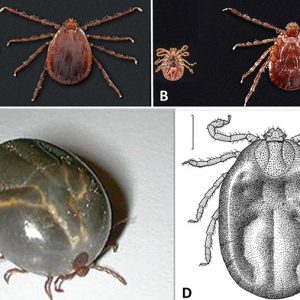
There are thirty-four species of Ixodes in the United States, with nine occurring in Arkansas, including Ixodes baergi on cliff swallows; I. banksi on muskrats; I. brunneus on migratory birds; I. cookei on small to medium-sized mammals (especially carnivores); I. dentatus on rabbits; I. marxi on squirrels; I. scapularis (black-legged tick) on medium-sized to large mammals including ungulates, carnivores, lagomorphs, and humans with immatures on the same hosts plus a wide range of small mammals, birds, and reptiles; I. texanus on raccoons; and I. woodi on woodrats. This genus of ticks contains no festoons, and the anal groove extends anterior to the anus. The females are often larger than males, and the mouthparts of females are more elongate than those of the males. Amblyomma is another important genus of tick in Arkansas. Festoons are present, the anal groove extends posterior to the anus, and the secondary palpal segment is elongate. Adults of Amblyomma americanum (the lone star tick) parasitize a variety of medium-sized to large mammals, especially white-tailed deer (Odocoileus virginianus), whereas immatures feed on various birds and mammals. It is the second-most-abundant tick infesting domestic dogs in Arkansas. Female A. americanum have a white or pink spot at the base of the scutum, and males are smaller than females with two inverted half circles at margin of the scutum. Another species, Amblyomma maculatum (the Gulf Coast tick), feeds on a variety of large mammals, such as deer, cattle, and humans, whereas immatures feed on smaller mammals and on birds.
Ticks of the genus Dermacentor have festoons, the anal groove extends posterior to the anus, and all three palp segments are short. Two species occur in the state, including the winter tick (Dermacentor albipictus) and the American dog tick (D. variabilis). D. albipictus is dark brown to gray with little ornamentation; is a one-host tick parasitizing a variety of ungulates including cattle, horses, and white-tailed deer; and is widely distributed throughout North America in the fall; however, there are relatively few records regarding this tick in Arkansas. D. variabilis is the most common tick encountered in the state. There is pronounced variegated white ornamentation on its scutum.
There is only one species of Haemaphysalis known from the state, the rabbit tick (H. leporispalustris). Adults mainly parasitize rabbits (Sylvilagus spp.), whereas immatures parasitize rabbits and a wide variety of ground-frequenting bird species. Festoons are present, the anal groove extends posterior to the anus, eyes are absent, and the secondary palp segment is flared laterally in this tick.
The brown dog tick, Rhipicephalus sanguineus (senso lato), is a non-native invasive tick and the most widely distributed tick in the world. In North America, all active stages typically feed on domestic dogs and appear to be the most common tick parasitizing domestic dogs in Arkansas. In this tick, festoons and eyes are present, the anal groove extends posterior to the anus, and the basis capitulum is laterally pointed so that it appears hexagonal. This tick tends to be brown with indistinct ornamentation.
A typical disease transmitted by soft ticks is relapsing fever (caused by Borrelia recurrentis), especially by Ornithodoros hermsi. Many diseases can be transmitted by hard ticks. Babesiosis (from a protozoan, Babesia spp.) is transmitted by multiple species and genera of ticks, depending upon the protozoan species involved. In cattle, east coast fever (Theileria parva) can be transmitted by Rhipicephalus appendicularis in Africa. Ehrlichiosis (from a bacterium, Ehrlichia spp.) is transmitted by multiple species and genera of ticks, depending upon the bacterium species. The Lyme disease bacterium (Borrelia burgdorferi) is transmitted especially by Ixodes scapularis. Q-fever (from a rickettsia) is a respiratory infection typically transmitted by Dermacentor spp. Rocky Mountain spotted fever (caused by Rickettsia rickettsii) is transmitted especially by Dermacentor variabilis in Arkansas and also Haemaphysalis leporispalustris between rabbits and Rhipicephalus sanguineus between canids. Texas cattle fever is caused by a protistan, Babesia bigemina, which is transmitted by Rhipicephalus (Boophilus) annulatus; this tick was the target of a federal tick eradication program in Arkansas in the early twentieth century. Tularemia (caused by the bacterium Francisella tularensis) is transmitted especially by Dermacentor andersoni and other Dermacentor spp. Finally, tick paralysis is caused by female ticks of certain species (e.g., D. variabilis in Arkansas) that release a neurotoxin during feeding that can induce a gradual, but reversible, paralysis.
Concerning specific tickborne diseases in Arkansas, the state ranked first in 2010 among U.S. states for reported cases of tularemia (caused by Francisella tularensis), fourth for Rocky Mountain spotted fever (RMSF) (caused by Rickettsia rickettsii), and tenth in reported cases of Human Monocytic Ehrlichiosis (HME) (caused by Ehrlichia chaffeensis). In addition, a cluster of zoonotic pathogens—Spotted Fever Group Rickettsiae (SFGR), ehrlichiae, and Borrelia burgdorferi—has been detected in ticks at Fort Chaffee in Sebastian County. However, from other research using DNA sequences, SFGR were detected in five species of ticks from Arkansas identified as Rickettsia montanensis and candidatus Rickettsia amblyommii but not R. rickettsia, suggesting that some Arkansas cases diagnosed as RMSF may actually represent infections with other rickettsial agents. As recently as March 2017, the Arkansas Department of Health discovered two cases of Lyme disease in the state. Previously, no cases that met the surveillance definition for the disease had been found in the state for nine years.
Tick species occurring in Arkansas that have the potential and/or have been found to transmit various pathogens are as follows: A. americanum is a vector of F. tularensis, HME, a form of granulocytic ehrlichiosis caused by Ehrlichia ewingii, Southern Tick Associated Rash Illness (STARI) (possible agent, Borrelia lonestari), American boutonneuse fever caused by Rickettsia parkeri, and feline cytauxzoonosis caused by Cytauxzoon felis; A. maculatum is a vector for the causative agent of American boutonneuse fever, R. parkeri, and its attachment can also cause a disfiguring condition called gotch ear in livestock animals that can lead to secondary bacterial infections, and R. parkeri and Candidatus Rickettsia amblyommii; D. variabilis is the primary vector for the causative agents of RMSF and tularemia; H. leporispalustris is an enzootic vector for the causative agents of tularemia and RMSF; I. cookei is a vector of Powassan virus; I. dentatus is a vector of Borrelia andersoni, which is closely related to the agent of Lyme disease; I. scapularis is the most important vector in eastern North America of the causative agents of Lyme disease, human granulocytic anaplasmosis (HGA) (caused by Anaplasma phagocytophilum), babesiosis (mainly caused by Babesia microti), and other pathogens including deer tick virus, a variant of Powassan virus that causes Powassan encephalitis; and R. sanguineus is the main vector of Babesia canis, a causative agent of canine babesiosis, and of Ehrlichia canis, a causative agent of canine ehrlichiosis (canine tropical pancytopenia). Other pathogens or symbionts transmitted to dogs by R. sanguineus in North America include R. rickettsii, Rickettsia rhipicephali (a non-pathogenic SFGR), Hepatozoon canis (causative agent of canine hepatozoonosis), Haemobartonella canis (an epierythrocytic rickettsial parasite), F. tularensis, and Coxiella sp. This ectoparasite can also (rarely) cause tick paralysis in dogs and recently was shown to be a vector of R. rickettsii to humans in Arizona.
One of the largest curated tick collections in the world, if not the largest, is the U.S. National Tick Collection (USNTC) at Georgia Southern University (GSU) in Statesboro, Georgia. It has well over 125,000 accessioned lots and more than one million specimens, with associated collection data and an extensive library (reprints, monographs, and books); it is owned and curated by the U.S. National Museum of Natural History (Smithsonian Institution) and has been housed at GSU since 1990. The collection contains specimens from all over the world and includes most of the approximately 860 known species of ticks, including Arkansas specimens.
For additional information:
Baerg, William J. “Ticks and Other Parasites Attacking Northern Cliff Swallows.” Auk 61 (1944): 413–414.
Calhoun, E. L. “Natural Occurrence of Tularemia in the Lone Star Tick, Ambylomma americanum (Linn.), and the Dogs in Arkansas.” American Journal of Tropical Medicine and Hygiene 3 (1954): 360–366.
Campbell, Haylee. “Survey of Ticks and Tick-Borne Pathogens Associated with Feral Swine (sus scrofa) in Arkansas.” MS thesis, University of Arkansas, 2021. Online at https://scholarworks.uark.edu/etd/4062/ (accessed July 6, 2022).
Cooley, Robert A., and Glen M. Kohls. “The Argasidae of North America, Central America and Cuba.” American Midland Naturalist Monograph 1(1944): 1–152.
———. “The Genus Amblyomma (Ixodidae) in the United States.” Journal of Parasitology 30 (1944): 77–111.
———. “Ixodes baergi, a New Species of Tick from Arkansas (Acarina: Ixodidae).” Public Health Reports (U. S. Public Health Service) 57 (1942): 1869–1872.
Durden, Lance A., and James E. Keirans. Nymphs of the Genus Ixodes (Acari: Ixodidae) in the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Lanham, MD: Entomological Society of America, 1996.
Durden, Lance A., James E. Keirans, and James H. Oliver. “The U.S. National Tick Collection: A Vital Resource for Systematics and Human and Animal Welfare.” American Entomologist 42 (1996): 239–243.
Ellis, H. R. “Common Ticks in Arkansas.” Journal of the Arkansas Medical Society 71 (1975): 380–382.
Goddard, J., and C. P. McHugh. “Impact of a Severe Tick Infestation at Little Rock AFB, Arkansas on Volant Scorpion Military Training.” Military Medicine 155 (1990): 277–280.
Kardatke, J. T., K. Neidhardt, D. P. Dzuban, J. L. Sanchez, and A. F. Azad. “Cluster of Tick-Borne Infections at Fort Chaffee, Arkansas: Rickettsiae and Borrelia burgdorferi in Ixodid Ticks.” Journal of Medical Entomology 29 (1992): 669–672.
Koch, H. G. “Seasonal Incidence and Attachment Sites of Ticks (Acari: Ixodidae) on Domestic Dogs in Southeastern Oklahoma and Northwestern Arkansas, USA.” Journal of Medical Entomology 19 (1982): 293–298.
Lancaster, Jay L., Jr. “Biology and Seasonal History of the Lone Star Tick in Northwest Arkansas.” Journal of Economic Entomology 48 (1955): 295–297.
———. “Checklist of the Ticks of Arkansas.” Arkansas Academy of Science, Arkansas Biota Survey Checklist 23. (1979).
———. “Control of the Lone Star Tick.” University of Arkansas (Fayetteville) Agricultural Experiment Station Report Series 67 (1957): 1–16.
———. “A Guide to the Ticks of Arkansas.” University of Arkansas (Fayetteville) Agricultural Experiment Station Bulletin 779 (1973): 1–39.
———. “The Lone Star Tick, Amblyomma americanum: A Contribution toward a Monograph of the Ticks of Arkansas.” Proceedings of the Arkansas Academy of Science 10 (1957): 38–43. Online at http://libraries.uark.edu/aas/issues/1957v10/v10a8.pdf (accessed June 21, 2017).
McAllister, Chris T., Matthew B. Connior, and Lance A. Durden. “Ectoparasites of Sciurid Rodents in Arkansas, Including New State Records for Neohematopinus spp. (Phthiraptera: Anoplura: Polyplacidae).” Journal of the Arkansas Academy of Science 67 (2013): 197–199. Online at http://libraries.uark.edu/aas/issues/2013v67/v67a32.pdf (accessed June 21, 2017).
McAllister, Chris T., Lance A. Durden, Matthew B. Connior, and Henry W. Robison. “Parasitism of Reptiles by the Blacklegged Tick (Ixodes scapularis) and Western Blacklegged Tick (Ixodes pacificus) with New Records of I. scapularis from Arkansas and Oklahoma Lizards: Implications for Lyme Disease Epidemiology.” Herpetological Review 44 (2013): 572–579.
McAllister, Chris T., Lance A. Durden, and Henry W. Robison. “The Ticks (Arachnida: Acari: Ixodida) of Arkansas.” Journal of the Arkansas Academy of Science 70 (2016): 141–154.
Richardson, Dennis J., Lance A. Durden, and Daniel E. Snyder. “Ectoparasites of the Raccoon (Procyon lotor) from North-Central Arkansas.” Journal of the Kansas Entomological Society 67 (1994): 208–212.
Schwartz, B. S., J. L. Sanchez, M. L. Sanders, and R. F. DeFraites. “Tick Avoidance Behaviors Associated with a Decreased Risk of Anti-Tick Salivary Gland Protein Antibody Seropositivity in Military Personnel Exposed to Amblyomma americanum in Arkansas.” American Journal of Tropical Medicine and Hygiene 55 (1996): 410–416.
Simpson, K. K., and Lawrence W. Hinck. “The Prevalence of Borrelia burgdorferi, the Lyme Disease Spirochete, in Ticks and Rodents in Northeast Arkansas.” Proceedings of the Arkansas Academy of Science 47 (1993): 110–115. Online at http://libraries.uark.edu/aas/issues/1993v47/v47a25.pdf (accessed June 21, 2017).
Sonenshine, Daniel E. Biology of Ticks. 2 vols. New York: Oxford University Press, 1991, 1993.
Trout, Rebecca T., and C. Dayton Steelman. “Ticks (Acari: Ixodidae) Parasitizing Canines and Deer in Arkansas.” Journal of Entomological Science 45 (2010): 140–149.
Trout, Rebecca T., C. Dayton Steelman, and Allen L. Szalanski. “Population Genetics and Phylogeography of Ixodes scapularis from Canines and Deer in Arkansas.” Southwestern Entomologist 34 (2009): 273–287.
———. “Population Genetics of Amblyomma americanum (Acari: Ixodidae) Collected from Arkansas.” Journal of Medical Entomology 47 (2010): 152–161.
Trout, Rebecca T., C. Dayton Steelman, Allen L. Szalanski, and K. Loftin. “Establishment of Amblyomma maculatum (Gulf Coast tick) in Arkansas, USA.” Florida Entomologist 93 (2010): 120–122.
Trout, Rebecca T., C. Dayton Steelman, Allen L. Szalanski, and Phillip C. Williamson. “Rickettsiae in Gulf Coast Ticks, Arkansas, USA.” Emerging Infectious Diseases 16 (2010): 830–832.
Trout Fryxell, Rebecca T., C. Dayton Steelman, Allen L. Szalanski, Ken L. Kvamme, Peggy M. Billingsley, and Phillip C. Williamson. “Survey of Borreliae in Ticks, Canines, and White-Tailed Deer from Arkansas, USA.” Parasites and Vectors 5 (2012): 139.
Tugwell, P., and Jay L. Lancaster Jr. “Results of a Tick-Host Study in Northwest Arkansas.” Journal of the Kansas Entomological Society 35 (1962): 202–211.
———.“Notes on the Seasonal Occurrence of Six Tick Species in Northwest Arkansas.” Journal of the Kansas Entomological Society 36 (1963): 167.
Washburn A, M., and J. M. Tuohy. “The Changing Picture of Tularemia Transmission in Arkansas.” Southern Medical Journal 42 (1949): 60–62.
Chris T. McAllister
Eastern Oklahoma State College








Comments
No comments on this entry yet.