calsfoundation@cals.org
Tickborne Diseases
Ticks are a very specific cosmopolitan collection of obligate, haematophagous, ectoparasitic arthropods of vertebrates (mostly on reptiles, birds, and mammals). They are important as vectors of bacterial (mainly rickettsial and spirochaetal), protistal, and viral disease agents of domestic animals and humans, as well as other mammals. By 2016, sixteen tickborne diseases of humans were known, which includes four emerging diseases discovered since 2013. In 2017, state and local health departments around the country reported a record number (totaling 59,349) of cases of tickborne diseases to the Centers of Disease Control and Prevention (CDC) in Atlanta, Georgia, up from 48,610 in 2016.
Tickborne diseases can range from producing mild symptoms that are treatable at home to causing symptoms including fever and severe infections, necessitating hospitalization and specific treatment. Although usually easily treated with broad-spectrum and more specific antibiotics, these diseases are often difficult for healthcare professionals to diagnose. However, in order to decrease the risk of serious complications, early recognition and treatment of the infection is crucial. Individuals should consult their physician immediately if they have been bitten by a tick and experience any of the symptoms described. Delay in treatment could result in severe illness and possibly death.
In the United States, Arkansas ranks at or near the top among states for three major tickborne diseases. The state ranked first in 2010 for reported cases of tularemia; fourth for Rocky Mountain spotted fever (RMSF); and tenth in reported cases of human monocytic ehrlichiosis (HME). In addition, a cluster of zoonotic pathogens—including spotted fever group rickettsiae (SFGR), ehrlichiae, and Borrelia burgdorferi—was detected in ticks at Fort Chaffee Joint Maneuver Training Center in Sebastian County. Using molecular techniques (DNA sequences), SFGR was identified in five species of ticks from Arkansas, and the causative agents were identified as Rickettsia montanensis and R. candidatus. However, some cases in the state diagnosed as RMSF may actually represent infections with other rickettsial agents. By March 2017, the Arkansas Department of Health (ADH) had discovered two cases of Lyme disease, which is tickborne, in the state. Interestingly, no cases that met the surveillance definition for the disease had been found in the state for the previous nine years. Tick species occurring in Arkansas have the potential to transmit various pathogens. For example, various ticks in the state are capable of transmitting anaplasmosis, babesiosis, and southern tick-associated rash illness (STARI).
Rocky Mountain Spotted Fever
The most common tickborne disease in Arkansas is Rocky Mountain spotted fever. A total of 6,248 cases of RMSF were reported to the CDC in 2017. It is caused by Rickettsia rickettsii, a gram-negative, intracellular, coccobacillus bacterium. RMSF is transmitted especially by the American dog tick (Dermacentor variabilis), Rocky Mountain wood tick (Dermacentor andersoni), and also the rabbit or grouse tick (Haemaphysalis leporispalustris) among rabbits, and brown dog tick (Rhipicephalus sanguineus) among canids in Central and South America. The primary vector in Brazil is the Cayenne tick, Amblyomma cajennense. It also takes an infected tick four to six hours to spread the disease after attaching to the host. About half of all humans with RMSF do not even recall being bitten. Typical symptoms of RMSF include high fever, headache, and muscle pain. After the onset of fever and generally after two to five days, a small, flat, pink, non-itchy rash appears on the extremities. This rash varies widely from person to person in appearance, location, and time of onset. However, about ten percent of people with RMSF never develop a rash. A red to purple, spotted (petechial) rash of RMSF is usually not seen until the sixth day or later after onset of symptoms and occurs in 35–60 percent of patients with RMSF. Blood tests are required to diagnose RMSF properly, but treatment should begin as soon as symptoms and/or recent tick exposure suggest the disease. The antibiotics of choice for the treatment of all spotted fever infections are doxycycline or tetracycline.
Helvetica Spotted Fever
Helvetica spotted fever is caused by Rickettsia helvetica with Ixodes ricinus, the main European tick vector. It has been confirmed in ticks from France, Sweden, Switzerland, and Laos. Symptoms include small red spots, fever, muscle pain, headache, and respiratory problems. Broad-spectrum antibiotics are usually prescribed as therapy. As for this and all other rickettsial infections, the treatment of choice is doxycycline.
Rickettsia parkeri Rickettsiosis
Rickettsiosis is a gram-negative intracellular bacterium transmitted to humans by the Gulf Coast tick (Amblyomma maculatum). It has been reported from people in the southeastern and mid-Atlantic states, as well as parts of southern Arizona. Less severe than RMSF, it is almost always associated with an inoculation eschar (an ulcerated, necrotic lesion) at the site of tick attachment. Several days after the eschar appears, fever, headache, rash (a sparse maculopapular or papulovesicular eruptions on the trunk and extremities), and muscle aches can develop.
364D Rickettsiosis (Pacific Coast Tick Fever)
Pacific Coast tick fever is caused by Rickettsia phillipi and transmitted to humans by the Pacific Coast tick (Dermacentor occidentalis). The proteome is part of the Rickettsia prowazekii (strain Madrid E) and is an obligate intracellular gram-negative bacteria mostly found in arthropods, some of which cause mild to severe diseases in humans. This is a relatively new disease that has only been found in California.
Human Ehrlichiosis
Formerly called human monocytic ehrlichiosis or HME, human ehrlichiosis is transmitted by the Lone Star tick (Ambylomma americanum), reported primarily from the southeastern and south-central regions of the United States, and from the eastern seaboard extending westward to Texas. Human ehrlichiosis is caused by Ehrlichia chaffeensis and E. ewingii, both obligate intracellular gram-negative species. The disease was first recognized in 1986 from a patient infected at Fort Chaffee. The areas from which cases are reported correspond with the known geographic distribution of A. americanum, which is associated with the transmission of both E. chaffeensis and E. ewingii. Three states—Arkansas, Missouri, and Texas—account for 35 percent of all reported E. chaffeensis infections. Symptoms of this disease may appear up to ten days after a tick bite and in about 30 percent of patients (up to 60 percent of children), and, after the onset of fever, a macular to maculopapular to petechial rash will appear. As with other tickborne diseases of this type, treatment is with doxycycline.
Canine Ehrlichiosis (Canine Tropical Pancytopenia)
Canine ehrlichiosis is a tickborne disease of dogs, with R. sanguineus the main vector of Ehrlichia canis. It is an obligate intracellular bacterium and causative agent of canine ehrlichiosis. The disease it causes most commonly affects the monocytes of canids (any breed but German shepherds may be predisposed) present throughout the United States (most prominent in the South), Africa, Asia, and South America. Several reports of this disease in cats suggest that feline infection may occur, albeit rarely. There are variable symptoms in dogs affected, including anorexia, depression, loss of stamina, stiffness and reluctance to walk, edema of the limbs or scrotum, and coughing or dyspnea. For dogs of all ages, doxycycline is recommended.
Tularemia
Tularemia, also called rabbit fever, is caused by the aerobic, intracellular gram-negative coccobacillus bacterium Francisella tularensis. The disease is named after Tulare County in California, where the disease was first discovered in 1911. It is transmitted to humans and animals (rabbits, hares, and rodents) by D. variabilis, the wood tick (Dermacentor andersoni), and A. americanum. In addition, H. leporispalustris is an enzootic vector for the causative agents of tularemia. In addition to tick bite, other routes of infection include skin contact with infected animals, especially from hunting or skinning infected rabbits; ingestion of contaminated water; inhalation of contaminated dusts or aerosols; and via deer flies. There were 239 cases of the disease reported to the CDC in 2017. Tularemia occurs throughout the United States, and in the most common form of the disease, a skin ulcer appears at the site where the organism entered the body. The ulcer is accompanied by swelling of regional lymph glands in the armpit and inguinal regions. Although tularemia can be life-threatening (e.g., the pneumonic form is often lethal without treatment), most infections can be treated successfully with antibiotics, including ciprofloxacin, doxycycline, gentamicin, or streptomycin.
Anaplasmosis
Previously known as human granulocytic ehrlichiosis (HGE), anaplasmosis is caused by the bacterium Anaplasma phagocytophilum, a small gram-negative bacterium and obligate intracellular pathogen. It is transmitted to humans by bites primarily of two tick species—the blacklegged tick (Ixodes scapularis) in the northeastern and upper midwestern United States, and the western blacklegged tick (Ixodes pacificus) along the Pacific coast. There were 7,718 cases of anaplasmosis/ehrlichiosis reported to the CDC in 2017. Symptoms of anaplasmosis infection may appear up to ten days after a tick bite. Classic signs and symptoms of the disease include fever, a decreased number of leucocytes (white blood cells) and platelets, and abnormally elevated liver enzyme (alanine transaminase and alkaline phosphatase) levels. The most effective courses of treatment are the antibiotics doxycycline, levofloxacin, and rifampin.
Babesiosis
Babesiosis is caused by apicomplexan parasites that infect erythrocytes (red blood cells). It is transmitted by multiple species and genera of ticks, depending upon the protistan species involved, and is endemic in field mice, Peromyscus spp., and voles (Microtus spp.). There were 2,368 cases of babesiosis reported to the CDC in 2017. Most cases of human babesiosis (also called piroplasmosis) in the United States are caused by Babesia microti, an intraerythrocytic parasite transmitted by I. dammini and I. scapularis and found primarily in the northeastern and upper midwestern United States. It is also responsible for cases of Texas cattle fever, redwater fever, tick fever, and Nantucket fever. The former is caused by Babesia bigemina, which is transmitted by Rhipicephalus (Boophilus) annulatus. This vector was the target of a federal tick eradication program in Arkansas in the early twentieth century. Rhipicephalus sanguineus is the main vector of Babesia canis, a causative agent of canine babesiosis. In mild-to-moderate babesiosis, the treatment of choice is a combination of atovaquone and azithromycin. However, in severe babesiosis, the combination of clindamycin and quinine is preferred, and in life-threatening cases, exchange blood transfusion is performed.
Lyme Disease
The Lyme disease bacterium (Borrelia burgdorferi senso lato), a microaerobic, motile spirochete, was first discovered in 1975, isolated in 1981, and described from Old Lyme, Connecticut; it also exists in Asia and Europe. The disease is transmitted by I. scapularis (=I. dammini) in the northeastern United States and upper Midwest, I. pacificus along the Pacific Coast, I. ricinus in Europe, and I. persulcatus in Asia. There were 42,743 cases of the disease reported to the CDC in 2017. Ninety-five percent of the reported Lyme disease cases come from fourteen states in the northeastern, mid-Atlantic, and upper Midwestern states. According to the CDC case definition for the disease, Arkansas is considered a low-incidence state. That equates to fewer than ten confirmed cases per 100,000 people for the previous three reporting years. Until 2016, this bacterium was the only known cause of Lyme disease in North America; however, a relatively new species, Borrelia mayonii, found in the upper midwestern United States, is also known to cause the disease. It has been found in blacklegged ticks in Minnesota and Wisconsin. In Europe and Asia, B. afzelii and B. garinii, respectively, are the leading carriers of Lyme disease. Depending on the stage of infection, clinical presentation of the disease is best recognized for the specific bull’s-eye rash (also known as erythema chronicum migrans), which occurs in approximately 70–80 percent of infected persons and begins at the site of the tick bite. Symptoms of infection can also include cardiac arrhythmias, arthritis, arthralgia, cardiomyopathy, facial nerve palsy, meningitis, myocarditis, and neuropathies. If left untreated, infection can spread to joints, the heart, and the nervous system. However, most cases of Lyme disease can be treated successfully with antibiotics: amoxicillin in pregnant adults and in children and doxycycline in other adults.
Testing and correct interpretation for Lyme disease is rather complicated. The CDC currently recommends a two-step process when testing blood for evidence of antibodies against the Lyme disease bacteria. Novel tests may be developed in the future as alternatives to one or both steps of the two-step process, but before the CDC will recommend new tests, their performance must be demonstrated to be equal to or better than the existing procedure, and they must be approved by the Food and Drug Administration (FDA). Patients treated with appropriate antibiotics (doxycycline, amoxicillin, or cefuroxime axetil) in the early stages of Lyme disease usually recover rapidly and completely. However, people with certain neurological or cardiac forms of the illness may require intravenous antibiotic treatment with ceftriaxone or penicillin.
Southern Tick-Associated Rash Illness (STARI)
Also known as Masters disease, STARI is transmitted via bites from A. americanum, found in the southeastern and eastern United States, with the possible agent being Borrelia lonestari. The rash of STARI is virtually identical to that of Lyme disease, with a red, expanding “bull’s-eye” rash that develops within a week around the site of a tick bite. However, unlike Lyme disease, STARI has not been linked to any arthritic or neurological symptoms. It is not known whether antibiotic treatment is necessary or beneficial for patients with STARI; however, physicians will usually still prescribe them.
Tickborne Relapsing Fever (TBRF)
Borrelia duttonii, the source of TBRF, is transmitted to people from the bite of infected soft ticks, especially Ornithodoros hermsi. Historically, relapsing fever has been described since the days of the ancient Greeks. Tickborne relapsing fever is widely distributed and found primarily in Africa, Saudi Arabia, Spain, Asia, and certain areas of Canada and the western United States. By 2020, TBRF had been reported from fifteen states (but not yet in Arkansas), including Arizona, California, Colorado, Idaho, Kansas, Montana, Nevada, New Mexico, Ohio, Oklahoma, Oregon, Texas, Utah, Washington, and Wyoming. It is associated with humans sleeping in rodent-infested rustic cabins and vacation homes. In Texas, TBRF is frequently linked to visiting caves. Symptoms of this bacterial infection are recurring bouts of fever, headache, muscle and joint aches, and nausea. Antibiotics are the treatment for TBRF, with doxycycline, erythromycin, or tetracycline being the treatments of choice.
Hard Tick Relapsing Fever
Also called Borrelia miyamotoi disease, hard tick relapsing fever is a type of spirochete bacterium that is closely related to the bacterium that causes TBRF. First identified in ticks from Japan in 1995, B. miyamotoi has since been detected in two types of North American ticks, I. scapularis and I. pacificus. It can cause symptoms similar to, or even worse than Lyme disease, such as fever, headaches, and severe rashes. To date, there are no comprehensive studies to evaluate treatment regimens, but in some cases, patients were successfully treated with antibiotic dosages used for Lyme disease.
American Boutonneuse Fever
American boutonneuse fever is caused by Rickettsia parkeri, a gram-negative intracellular bacterium with A. maculatum as the vector for the causative agent. Its attachment to certain hosts can also cause a disfiguring condition called gotch ear in livestock animals that can lead to secondary bacterial infections. The first report of human infection with R. parkeri was made in 2002 for a forty-year-old patient from Virginia. The patient complained of arthralgias, fever, headache, malaise, myalgias, and (most notably) spots of necrosis (eschars) on his legs, where the ticks had presumably fed. Since then, several more cases of R. parkeri causing disease in humans have been observed and reported in Alabama, Florida, Kentucky, Mississippi, and some states on the eastern coastal areas of the United States. This illness can be treated with antibiotics such as tetracyclines (doxycycline is the preferred treatment), chloramphenicol, fluoroquinolones, or macrolides.
Colorado Tick Fever (CTFV)
CTFV is caused by a coltivirus (family Reoviridae) transmitted by D. andersoni. It occurs in the states comprising the Rocky Mountains at elevations ranging from 1,219 to 3,200 m (4,000 to 10,500 ft.). CTFV infects haemopoietic cells, particularly erythrocytes, which explains how the virus is transmitted by ticks and also explains the incidence of transmission via blood transfusion. Initial symptoms include chills, fever, headaches, pain behind the eyes, light sensitivity, muscle pain, generalized malaise, abdominal pain, hepatosplenomegaly, nausea and vomiting, and a flat or pimply rash. During the next phase of the virus, a high fever can return with an increase in symptoms. CTFV can be very severe in cases involving children and can require hospitalization. Complications with this disease are rare but have included aseptic meningitis, encephalitis, and hemorrhagic fever. Specific treatment for CTFV is not yet available.
Powassan Disease (POWV)
POWV is a flavivirus (a positive, single-stranded, enveloped RNA virus) transmitted by I. scapularis and the groundhog tick (Ixodes cookei). There were only thirty-three cases of the disease reported to the CDC in 2017, and those were reported primarily from the Great Lakes region, northeastern U.S. states, and the Russian Far East. Both ticks have been found on dogs in Arkansas. Other tick vectors include I. marxi, D. andersoni, and D. variabilis. The disease can cause encephalitis, an infection of the brain. A separate lineage (Lineage 2 POWV) is also known as deer tick virus (DTV). There is no medication (vaccine or antiviral drugs) to treat POWV infection.
Severe Febrile Disease (Heartland Virus)
Heartland virus is a tickborne phlebovirus of the Bhanja virus serocomplex discovered in 2009. By 2017, five states (Arkansas, Indiana, Missouri, Oklahoma, and Tennessee) had reported twenty human infections; symptoms resemble those of ehrlichiosis and anaplasmosis. Research suggests that Lone Star ticks can transmit the virus. The reservoir host is unknown, but deer, raccoons, coyotes, and moose in thirteen states have demonstrated positive antibody titers against the virus. Antibiotics are not useful for viruses, so treatment is non-specific. However, intravenous fluid administration and medications for the relief of pain are currently the best options.
Bourbon Virus
Infection from this RNA virus in the genus Thogotovirus of the family Orthomyxoviridae has been identified in a limited number of patients in the midwestern and southern United States. However, relatively little is known about the virus. It was first identified in 2014 in a patient from Bourbon County, Kansas, who died after being bitten by ticks. In May 2015, a case was discovered in a patient who fully recovered in Stillwater, Oklahoma. In June 2017, a fifty-eight-year-old woman in Missouri died from an infection of Bourbon virus after it had been misdiagnosed for a significant period of time. The virus is suspected to be transmitted by ticks or other insects, and common-sense avoidance of bites is recommended to reduce risk of infection. No specific treatment or vaccine is available.
Crimean-Congo Hemorrhagic Fever (CCHF)
CCHF is caused by a nairovirus (Bunyaviridae) with the vectors being the ticks Hyalomma marginatum and Rhipicephalus bursa. It occurs in people living in southern Asia, northern Africa, and southern Europe. Symptoms of CCHF may include fever, diarrhea, headache, muscle pain, vomiting, and bleeding into the skin, with major complications including liver failure. In patients who survive, recovery generally occurs about two weeks after onset.
Tick-Borne Meningoencephalitis (TBE)
TBE was first isolated in 1937 and is a flavivirus of the family Flaviviridae, with I. scapularis as the vector in North America, I. ricinus in Europe, and I. persulcatus in Asia and Russia. It can be sometimes, though rarely, be transmitted through the non-pasteurized milk of infected cows. Long-lasting or permanent neuropsychiatric consequences are observed in 10–20 percent of infected patients. It is also known to infect a range of vertebrate hosts, including birds, rodents, carnivores, ruminants, horses, and humans. The disease can also be spread from animals to humans, with ruminants and dogs providing the principal source of infection for humans. Once manifested, the disease is incurable; therefore, there is no specific drug therapy for TBE, although anti-inflammatory drugs (corticosteroids) may be considered under specific circumstances for symptomatic relief. Eventually, symptomatic brain damage requires hospitalization, and supportive care based on syndrome severity with tracheal intubation and respiratory support may be necessary.
Tick Paralysis
Tick paralysis is caused by toxins in the salivary gland of female ticks of certain species, especially D. variabilis in Arkansas, but also D. andersoni in other regions of North America. In Australia, the vector is Ixodes holocyclus. The incidence of tick paralysis is unknown. Ticks release a neurotoxin during feeding that can induce a gradual, but reversible, paralysis. After prolonged attachment, the engorged tick transmits the toxin to its host. Patients can experience severe respiratory distress (similar to anaphylaxis). Diagnosis is based on symptoms and upon finding an embedded tick, usually on the scalp, and human cases are rare but those that do occur are usually in children under the age of ten.
Cytauxzoonosis
Cytauxzoonosis is caused by Cytauxzoon felis, an apicomplexan hemoparasite of domestic and wild mammals of the family Felidae. The disease was first reported in Missouri, and this emerging disease has since been identified in wild and domestic felid hosts from various states. It is transmitted by the Lone Star tick (Amblyomma americanum); D. variabilis is also implicated to carry the protistan, but its ability to transmit the disease may be limited. In North America, the sylvatic reservoir for C. felis is the bobcat (Felis rufus), in which the infection is apparently self-limiting. However, in domestic cats (F. catus) it causes a highly fatal disease, with a geographic distribution that covers the majority of the central, southcentral, and southeastern United States. The most-often-used effective treatments for cytauxzoonosis are imidocarb dipropionate and a combination of atovaquone and azithromycin.
Multiple Tickborne Infections
In ticks that are capable of transmitting B. burgdorferi (Lyme disease) to humans, several other parasites can also be multiply transmitted to people, such as T. microti (babesiosis) and A. phagocytophilum (HGE). For example, among patients with early Lyme disease, dependent on their geographic location, 2–40 percent will have babesiosis and 2–12 percent will also have HGE. This makes diagnosis and treatment very difficult and may complicate their Lyme disease.
For additional information:
Anderson, J. F., L. A. Magnarelli, Willy Burgdorfer, and A. G. Barbour. “Spirochetes in Ixodes dammini and Mammals from Connecticut.” American Journal of Tropical Medicine and Hygiene 32 (1982): 818‒824.
Burgdorfer, Willy. “Discovery of the Lyme Disease Spirochete and its Relation to Tick Vectors.” Yale Journal of Biology and Medicine 57 (1984): 515‒520.
Burgdorfer, Willy, A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. “Lyme Disease-a Tick-Borne Spirochetosis?” Science 216 (1982): 1317–1319. https://escholarship.org/uc/item/9vj3t37b (accessed June 11, 2020).
Calhoun, E. L. “Natural Occurrence of Tularemia in the Lone Star Tick, Ambylomma Americanum (Linn.), and the Dogs in Arkansas.” American Journal of Tropical Medicine and Hygiene 3 (1954): 360–366.
Campbell, Haylee. “Survey of Ticks and Tick-Borne Pathogens Associated with Feral Swine (sus scrofa) in Arkansas.” MS thesis, University of Arkansas, 2021. Online at https://scholarworks.uark.edu/etd/4062/ (accessed July 6, 2022).
Dworkin, M. S., P. C. Shoemaker, and D. Anderson. “Tick Paralysis: 33 Human Cases in Washington State, 1946–1996.” Clinical Infectious Diseases 29 (1999): 1435–1439.
Ebel, G. D., and L. D. Kramer. “Short Report: Duration of Tick Attachment Required for Transmission of Powassan Virus by Deer Ticks.” American Journal of Tropical Medicine and Hygiene 71 (2004): 268–271.
Ellis, H. R. “Common Ticks in Arkansas.” Journal of the Arkansas Medical Society 71 (1975): 380–382.
Goddard, J., and C. P. McHugh. “Impact of a Severe Tick Infestation at Little Rock AFB, Arkansas on Volant Scorpion Military Training.” Military Medicine 155 (1990): 277–280.
Goddard, J., and A. Varela-Stokes. “The Discovery and Pursuit of American Boutonneuse Fever: A New Spotted Fever Group Rickettsiosis.” MidSouth Entomologist 2 (2008): 47‒52.
Jacobs, Cynthia H. “Prevalence of Cytauxzoon felis (Protista: Apicomplexa) in Feral Cats in Russellville Arkansas.” Journal of the Arkansas Academy of Science 72 (2018): 123‒128. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=3305&context=jaas (accessed June 11, 2020).
Kardatke, J. T., K. Neidhardt, D. P. Dzuban, J. L. Sanchez, and A. F. Azad. “Cluster of Tick-Borne Infections at Fort Chaffee, Arkansas: Rickettsiae and Borrelia burgdorferi in Ixodid Ticks.” Journal of Medical Entomology 29 (1992): 669–672.
Koch, H. G. “Seasonal Incidence and Attachment Sites of Ticks (Acari: Ixodidae) on Domestic Dogs in Southeastern Oklahoma and Northwestern Arkansas, USA.” Journal of Medical Entomology 19 (1982): 293–298.
Kosoy, Olga I., Amy J. Lambert, Dana J. Hawkinson, Daniel M. Pastula, Cynthia S. Goldsmith, D. Charles Hunt, and J. Erin Staples. “Novel Thogotovirus Associated with Febrile Illness and Death, United States, 2014.” Emerging Infectious Diseases 21 (2015): 760–764. Online at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4412252/ (accessed June 11, 2020).
Krause, P. J., S. Narasimhan, G. P. Wormser, A. G. Barbour, A. E. Platonov, and J. Brancato, et al. “Borrelia miyamotoi Sensu Lato Seroreactivity and Seroprevalence in the Northeastern United States.” Emerging Infectious Diseases 20 (2014): 1183‒1190. Online at https://wwwnc.cdc.gov/eid/article/20/7/13-1587_article (accessed June 11, 2020).
Muehlenbachs, A., C. R. Fata, A. J. Lambert, C. D. Paddock, J. O. Velez, D. M. Blau, J. E. Staples, M. B. Karlekar, J. Bhatnagar, R. S. Nasci, and S. R. Zaki. “Heartland Virus Associated Death in Tennessee External Icon.” Clinical Infectious Diseases 15 (2014): 845‒850. Online at https://pubmed.ncbi.nlm.nih.gov/24917656/ (accessed June 11, 2020).
Lancaster, Jay L., Jr. “Biology and Seasonal History of the Lone Star Tick in Northwest Arkansas.” Journal of Economic Entomology 48 (1955): 295–297.
———. “Checklist of the Ticks of Arkansas.” Arkansas Academy of Science, Arkansas Biota Survey Checklist 23, 1979.
———. “Control of the Lone Star Tick.” University of Arkansas (Fayetteville) Agricultural Experiment Station Report Series 67 (1957): 1–16.
———. “A Guide to the Ticks of Arkansas.” University of Arkansas (Fayetteville) Agricultural Experiment Station Bulletin 779 (1973): 1–39.
———. “The Lone Star Tick, Amblyomma americanum: A Contribution toward a Monograph of the Ticks of Arkansas.” Proceedings of the Arkansas Academy of Science 10 (1957): 38–43. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=3238&context=jaas (accessed June 11, 2020).
McAllister, Chris T., Lance A. Durden, Matthew B. Connior, and Henry W. Robison. “Parasitism of Reptiles by the Blacklegged Tick (Ixodes scapularis) and Western Blacklegged Tick (Ixodes pacificus) with New Records of I. scapularis from Arkansas and Oklahoma Lizards: Implications for Lyme Disease Epidemiology.” Herpetological Review 44 (2013): 572–579.
McAllister, Chris T., Lance A. Durden, and Henry W. Robison. “The Ticks (Arachnida: Acari: Ixodida) of Arkansas.” Journal of the Arkansas Academy of Science 70 (2016): 141–154. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2195&context=jaas (accessed June 11, 2020).
Paulissen, Leo J., E. Reece Corey, and Delbert Swartz. “Tularemia in the Wildlife of Arkansas.” Journal of the Arkansas Academy of Science: 21 (1967): 39‒44. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=3067&context=jaas (accessed June 11, 2020).
Rizzi, T. E., M. V. Reichard, L. A. Cohn, A. J. Birkenheuer, J. D. Taylor, and J. H. Meinkoth. “Prevalence of Cytauxzoon felis Infection in Healthy Cats from Enzootic Areas in Arkansas, Missouri, and Oklahoma.” Parasites & Vectors 8 (2015): 13.
Schwartz, B. S., J. L. Sanchez, M. L. Sanders, and R. F. DeFraites. “Tick Avoidance Behaviors Associated with a Decreased Risk of Anti-Tick Salivary Gland Protein Antibody Seropositivity in Military Personnel Exposed to Amblyomma americanum in Arkansas.” American Journal of Tropical Medicine and Hygiene 55 (1996): 410–416.
Simpson, Kim K., and Lawrence W. Hinck. “The Prevalence of Borrelia burgdorferi, the Lyme Disease Spirochete, in Ticks and Rodents in Northeast Arkansas.” Proceedings of the Arkansas Academy of Science 47 (1993): 110–115. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2027&context=jaas (accessed June 11, 2020).
Sonenshine, Daniel E. Biology of Ticks. 2 vols. New York: Oxford University Press, 1991, 1993.
Strickland, R. K., R. R. Gerrish, J. L. Hourrigan, and G. O. Schubert. Ticks of Veterinary Importance. APHIS, U.S. Department of Agriculture Agricultural Handbook No. 485, 1976.
“Tickborne Diseases of the United States.” Centers for Disease Control and Prevention. https://www.cdc.gov/ticks/tickbornediseases/index.html (accessed June 11, 2020).
Trout, Rebecca T., and C. Dayton Steelman. “Ticks (Acari: Ixodidae) Parasitizing Canines and Deer in Arkansas.” Journal of Entomological Science 45 (2010): 140–149.
Trout, Rebecca T., C. Dayton Steelman, Allen L. Szalanski, and Phillip C. Williamson. “Rickettsiae in Gulf Coast Ticks, Arkansas, USA.” Emerging Infectious Diseases 16 (2010): 830–832.
Trout Fryxell, Rebecca T., C. Dayton Steelman, Allen L. Szalanski, Ken L. Kvamme, Peggy M. Billingsley, and Phillip C. Williamson. “Survey of Borreliae in Ticks, Canines, and White-Tailed Deer from Arkansas, USA.” Parasites and Vectors 5 (2012): 139.
Washburn A, M., and J. M. Tuohy. “The Changing Picture of Tularemia Transmission in Arkansas.” Southern Medical Journal 42 (1949): 60–62.
Chris T. McAllister
Eastern Oklahoma State College
 Health and Medicine
Health and Medicine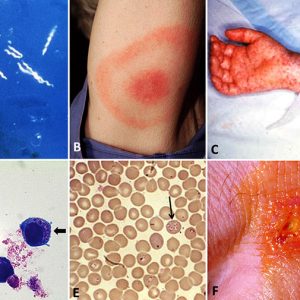 Tickborne Diseases Examples
Tickborne Diseases Examples 




Comments
No comments on this entry yet.