calsfoundation@cals.org
Tardigrades
aka: Water Bears
aka: Moss Piglets
Tardigrades (sometimes called water bears or moss piglets) are microscopic members of the Phylum Tardigrada, numbering more than sixty-seven subspecies, 1,018 species, four subgenera, 105 genera, fifteen subfamilies, twenty families, five orders, and three classes. Of these, there are fifty-four genera and 380 species known from the Americas, 245 species from the Nearctic ecozone, and 251 in the Neotropical ecozone. Several species of tardigrades can be found in Arkansas.
Discovered in 1773 by the German entomologist and pastor Johann August Ephraim Goeze (1731–1793), they were nicknamed “water bears” because of their plump, bear-like appearance; legs with claws; and slow, lumbering gait. The name Tardigrada (“slow steppers”) was given in 1777 by the Italian Catholic priest, biologist, and physiologist Lazzaro Spallanzani (1729–1799).
The very minute size of tardigrades and their membranous integuments make their fossilization problematic to detect and highly infrequent in occurrence. The only known fossil specimens are those from mid-Cambrian deposits in Siberia, Russia, and a few rare specimens from Cretaceous amber have been found in two North American locations.
Scientists have conducted both conventional morphological and higher-level molecular studies to unravel how tardigrades relate to other lineages of ecdysozoan (molting) organisms. Tardigrades are a sister group of Lobopodia and are thought to be related to the lineage of invertebrates in the Phyla Arthropoda (crustaceans, spiders, and insects) and Onychophora (velvet worms), and are often referred to as a “lesser known taxa.” The phylum is divided into three classes: Heterotardigrada, Mesotardigrada, and Eutardigrada. Their numbers have approximately doubled from 1990 to 2020 due to new explorations, better methodologies, revised taxonomic criteria, and revisions of previously known taxa.
Tardigrades have been found from polar regions to the equator across the earth’s surface—on mountaintops (at 6,000 m [20,000 ft.]), in the deep seas (at −4,000 m [−13,000 ft.]), in mud volcanoes, in tropical rainforests, and in the Antarctic. Scientists have found tardigrades in hot springs and under layers of solid ice, and in ocean sediments. Other environments include beaches, dunes, leaf litter, soil, and marine or freshwater and limnoterrestrial sediments. Tardigrades have even been found in holes in glaciers. Many species are collected from cryptogams (mosses, lichens, and liverworts). The greatest number of tardigrades has been described from terrestrial habitats where they are inactive unless covered by a film of water. The least number of tardigrades is found in limnic habitats, and some species can live in both terrestrial and freshwater habitats. Interestingly, the first exposure to tardigrades often comes to students in general biology laboratories when they explore those found in local moss samples.
Tardigrades are among the most resilient animals known to science and are able to survive in extreme environments that would kill almost any other organism. They can survive under severe conditions for centuries, but all tardigrades eventually require moist environments for life processes. For example, scientists have records of tardigrades regenerated from dried moss kept in a museum for over 100 years. Once the moss was moistened, live tardigrades were recovered. In addition, they can survive under life-threatening dry conditions in the Sahara Desert, in extreme cold conditions where they can survive at -273 °C (-459.4 °F), or even in the vacuum of space and under radiation. Research being conducted on tardigrades in the Ikka Fjord of Greenland, where the unique Ikkaite Tufa columns made of calcium carbonate hexahydrate originating from alkaline cold springs at the bottom of the fjord creates very specific environments with nearly brackish conditions in the middle and marine water salinity on the outside. As such, this creates varied conditions for different species of Tardigrada.
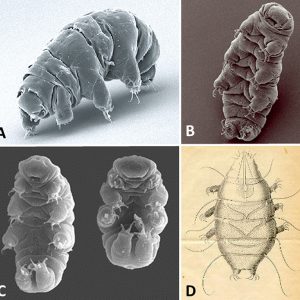
Tardigrades are hydrophilous micrometazoans with a bilaterally symmetrical body and four pairs of legs ending in four to eight claws. Adults range in size from 0.1 mm to 1.5 mm (0.004 to 0.06 in.) in length. They are convex on the dorsal side and flattened on the ventral side. They can be divided into a head (cephalic segment), three trunk segments each with a pair of legs, and a caudal segment with a posteriorly directed fourth pair of legs. The body is covered by a cuticle that contains chitin and protein and is molted periodically. Coloration is typically translucent or whitish. Interestingly, all adult tardigrades of the same species have the same number of cells. Some species have as many as 40,000 cells in each adult, while others have far fewer.
Their body cavity consists of a hemocoel, with a true coelom only occurring around the gonads. Tardigrades have no respiratory organs; thus, gas exchange occurs across the entire body. While some tardigrades have three tubular glands associated with the rectum, these may be excretory organs similar to the Malpighian tubules of arthropods. The head region has a tubular mouth armed with stylets that are used to pierce plant cells or small invertebrates, thereby releasing the cell contents or body fluids. The mouth is connected to a triradiate, muscular pharynx. When the animal molts, the stylets are lost, and a new pair is secreted from a pair of glands that lie on either side of the mouth. Tardigrades all possess a buccopharyngeal apparatus (swallowing device) composed of muscles and spines that activates an inner jaw and begins digestion and movement along the throat and intestine. This structure, together with the claws, is used to differentiate various species. The pharynx is connected directly to a short esophagus and then to an intestine where digestion occurs. The intestine is followed by a short rectum and an anus located at the posterior tip of the body.
The nervous system of tardigrades consists of a brain with multiple lobes, primarily consisting of three bilaterally paired clusters of neurons. The actual brain is attached to a large ganglion below the esophagus from which a double ventral nerve cord runs the length of the body. The ventral nerve cord has one ganglion per segment, and each of them produces lateral nerve fibers that run into the limbs. Their sensory apparatus includes a pair of rhabdomeric pigment-cup eyes and numerous sensory bristles on the head and body.
Tardigrades have a digestive system composed of a foregut, midgut, and hindgut. The foregut and hindgut have a cuticular lining, while the hindgut is subdivided into an anterior hindgut (rectum) and a true cloaca in the eutardigrades; in the heterotardigrades, the reproductive and digestive systems are separated, the hindgut is a rectum, and the gonopore opens anteriorly to the anus. In terms of food habits, tardigrades feed on the fluid of bacterial, plant, and animal cells, and, in turn, they themselves become prey for amoebas and nematodes. Some species are entirely carnivorous and even cannibalistic.
Several life history strategies are utilized by tardigrades. Tardigrades reproduce via asexual (parthenogenesis) or sexual reproduction. Marine tardigrades are almost always bisexual (gonochoristic), and hermaphroditic species are quite rare. In gonochoric terrestrial and limnic species, the ratio of males to females may be equal. Usually, limnoterrestrial tardigrades are unisexual and composed of only females that reproduce parthenogenetically. In bisexual populations, cross-fertilization occurs to provide the benefit of genetic recombination due to the fusion of two different genomes. Self-fertilization has been observed in two species of eutardigrades. Hermaphroditism is rare in eutardigrades but does occur in the following species: Parhexapodibius pilatoi from grasslands, Macrobiotus joannae from moss, Bertolanius weglarskae from pine litter, and four species of Isohypsibius from freshwater. Sexual maturity is reached with the second or third molt. Molting requires five to ten days and occurs periodically (up to twelve times) throughout the life of a tardigrade. Typically, body length increases after each molt until a maximum size is attained.
Females lay from one to sixty eggs during each oviposition. After no more than two weeks, eggs hatch with the young already possessing their full complement of adult cells. Enlargement of the individual cells (hypertrophy) rather than by typical cell division is responsible for growth to the adult size. Egg production occurs throughout the entire life of an individual. Eggs can be found in lichens, mosses, and soil at any time of the year. Eggs are homolechithal, and the embryo undergoes an irregular indeterminate cleavage pattern without early fate determination. Parental care is quite limited in tardigrades and only found in limnic eutardigrade species. Lifespan has been estimated to be from three to thirty months, even up to three years.
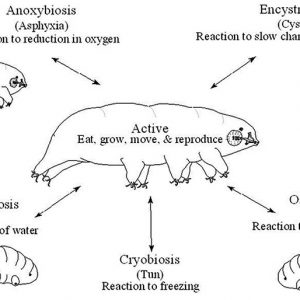
Tardigrades are known for their ability to enter cryptobiosis. Cryptobiosis may be defined as a metabolic state of life entered by an organism in response to adverse environmental conditions such as desiccation, freezing, and oxygen deficiency. While in this cryptobiotic state, all measurable metabolic processes stop, preventing reproduction, development, and even tissue repair. Cryptobiosis is a safeguard state for the animal because when environmental conditions return to being hospitable, the organism will return to its metabolic state of life as it was prior to the cryptobiosis. There are various types of cryptobiosis, including (1) anhydrobiosis (lack of water), (2) cryobiosis (low temperature), (3) osmobiosis (increased solute concentration, such as salt water), and (4) anoxybiosis (lack of oxygen). The most common type of cryptobiosis studied in tardigrades is anhydrobiosis.
Scientists know that tardigrades can survive dry periods by curling up into a little ball called a tun. For a tun to form requires metabolism and synthesis of a protective sugar known as trehalose that moves into the cells and replaces lost water. Tardigrades lower their metabolism to less than 0.01 percent of normal while in the tun stage. Typically, revival takes only a few hours, depending on how long the tardigrade has been in the cryptobiotic state.
Tardigrades are also famous for being able to survive freezing temperatures (cryobiosis) and then thawing, allowing them to exist in very cold polar regions. Frozen tardigrades can survive for several years. Experiments conducted on several species revealed that terrestrial tardigrades have a higher cryobiotic ability than limnic species. Some tardigrades that live in fresh water, mosses, and soil can encyst during harsh environmental conditions. Cysts have a distinctive contracted oval form with thick cuticular layers. Cyst formation actually involves complex morphological changes in tardigrades where the tardigrade becomes a dark, immobile, contracted oval shape with very retracted legs and modified claws. Encysted tardigrades have a metabolic rate lower than active individuals, but higher than anhydrobiotic ones, and they can survive for months in nature.
There are about 150 species of marine tardigrades that typically belong to the class Heterotardigrada in either the orders Arthrotardigrada or Echiniscoidea. Most marine tardigrades live in the intertidal zone and shallow waters off the continental shelf. The class Eutardigrada contains both freshwater (hydrophilous) species and terrestrial (xerophilous, eurytopic, and hygrophilous) species. Hydrophilous species are considered “distinctly aquatic” and live only in permanent freshwater habitats. Most species live in the littoral zone of lakes or ponds, although deeper water species have been found. Tardigrades are also known from subterranean caves as well as the hyporheic zone of groundwater. Limnoterrestrial tardigrades can live in both freshwater and terrestrial environments. Interestingly, the true limnic taxa of eutardigrades generally have long legs and very long claws. Four genera (Dactylobiotus, Macroversum, Pseudobiotus, and Thulinius) are exclusively limnic in habitat preference. Dactylobiotus can also be found on submerged plants and filamentous algae.
In a 2012 study in Arkansas, samples of moss and lichen growing on the bark of seven different species of trees on the University of Central Arkansas (UCA) campus in Conway (Faulkner County) were found to contain tardigrades. Five of the eleven genera of tardigrades previously reported from the state (45 percent), and five of the twenty-five previous reported species (20 percent) were found in the UCA campus samples. Two species (Milnesium eurystomum, Macrobiotus polyopus) constituted new records for the state, but one species of Echiniscus (arctomys group) could not be identified and may be new.
In a 2001 study, samples of mosses and lichens from trees and rocks were collected from three sites in central and southern Louisiana and six sites in western Arkansas. Leaf litter samples were collected from one site in Louisiana. Nine species of tardigrades were found in Louisiana and twenty-two in Arkansas. The number of species per sample ranged from one to six.
In terms of conservation efforts, to date, tardigrades have not been evaluated by the International Union for Conservation of Nature. According to the National Geographic Society, they are also not on any other endangered or threatened list and have survived five mass extinctions over the course of around half a billion years.
For additional information:
Bartels, P. J., and D. R. Nelson. “A Large-Scale, Multihabitat Inventory of the Phylum Tardigrada in the Great Smoky Mountains National Park, USA: A Preliminary Report.” Hydrobiologia 558 (2006): 111‒118.
Bertolani, R. “Evolution of the Reproductive Mechanisms in Tardigrades—A Review.” Zoologischer Anzeiger 240 (2001): 247‒252.
Blaxter, Mark, Ben Elsworth, and Jennifer Daub. “DNA Taxonomy of a Neglected Animal Phylum: An Unexpected Diversity of Tardigrades.” Proceedings of the Royal Society B: Biological Sciences 271 (2004): S189–S192.
Budd, Graham E. “Tardigrades as ‘Stem-Group Arthropods’: The Evidence from the Cambrian Fauna.” Zoologischer Anzeiger 240 (2001): 265–279.
Crowe, John H., John F. Carpenter, and Lois M. Crowe. “The Role of Vitrification in Anhydrobiosis.” Annual Review of Physiology 60 (1998): 73–103.
Goldstein, Bob. “The Emergence of the Tardigrade Hypsibius exemplaris as a Model System.” CSH Protocols 10 (2018): 859‒866.
Guidetti, R., and R. Bertolani. “Tardigrade Taxonomy: An Updated Checklist of the Taxa and a List of Characters Used in Their Identification.” Zootaxa 845 (2005): 1‒46.
Guidetti, R., T. Altiero, and L. Rebecchi. “On Dormancy Strategies in Tardigrades.” Journal of Insect Physiology 57 (2011): 567‒576.
Guil, N., S. Snachex-Moreno, and A. Machordom. “Local Biodiversity Patterns in Micrometazoans: Are Tardigrades Everywhere?” Systematics and Biodiversity 7 (2009): 259–268.
Kinchin, I. M. The Biology of the Tardigrades. London: Portland Press, 1994.
Land, Marshalluna, Adam Musto, William R. Miller, David E. Starkey, and Jeffrey D. Miller. “Tardigrades of the University of Central Arkansas Campus, Conway, AR.” Southeastern Naturalist 11 (2012): 469‒476.
McInnes, S. J. “Zoogeographic Distribution of Terrestrial/Freshwater Tardigrades from Current Literature.” Journal of Natural History 28 (1994): 257‒352.
McInnes, S. J., and D. B. Norman. “Tardigrade Biology.” Zoological Journal of the Linnean Society 1‒2 (1996): 1‒243.
Meyer, Harry A. “A Contribution to the Tardigrade Fauna of Oklahoma.” Proceedings of the Oklahoma Academy of Sciences 89 (2009): 55–58.
———. “Distribution of Tardigrades in Florida.” Southeastern Naturalist 7 (2008): 91‒100.
———. “Interspecific Association and Substrate Specificity of Tardigrades from Florida, Southeastern United States.” Hydrobiologica 558 (2006): 129‒132.
———. “Tardigrades of Louisiana and Arkansas, United States of America.” Zoologischer Anzeiger 240 (2001): 471–474.
———. “Terrestrial and Freshwater Tardigrada of the Americas.” Zootaxa 3747 (2013): 1‒71.
Miller, Stephen A., and Todd A. Tupper. Zoology, 11th ed. New York: McGraw-Hill Education, 2019.
Miller, William R. “Tardigrades: Bears of the Moss.” Kansas School Naturalist 43 (1997): 1–16.
———. “Tardigrades.” American Scientist 99 (2011): 384.
Nelson, Diane R. “Current Status of Tardigrada: Evolution and Ecology.” Integrative and Comparative Biology 42 (2002): 652–659.
———. Proceedings of the Third International Symposium on Tardigrades. August 3–6, 1980. Johnson City: East Tennessee State University Press, 1982.
Smith, Frank W., Thomas C. Boothby, Ilaria Giovannini, Lorena Rebecchi, Elizabeth L. Jockusch, and Bob Goldstein. “The Compact Body Plan of Tardigrades Evolved by the Loss of a Large Body Region.” Current Biology 26 (2016): 224–229.
Tsujimoto, Megumu, Satoshi Imura, and Hiroshi Kanda. “Recovery and Reproduction of an Antarctic Tardigrade Retrieved from a Moss Sample Frozen for Over 30 Years.” Cryobiology 72 (2015): 78–81.
Zhang, Zhi-Qiang. “Animal Biodiversity: An Introduction to Higher-Level Classification and Taxonomic Richness.” Zootaxa 3148 (2011): 7–12.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College





Comments
No comments on this entry yet.