calsfoundation@cals.org
Earwigs
Earwigs belong to the Phylum Arthropoda, Class Insecta, and Order Dermaptera. It is one of the comparatively species‐poor insect orders, as there are about 2,200 extant species within eleven families. About twenty-five species occur in North America, sixty in Australia, and forty-five in Europe. Earwigs are found on all continents except Antarctica and occur in northern latitudes as far north as Greenland. Earwigs exhibit their major diversity in the tropics and have a strong preference for warm and moist environments; few survive winter outdoors in cold climates. The overwhelming majority of earwig species are in the suborder Forficulina, grouped into nine families of 180 genera, including the common European earwig, Forficula auricularia. As of 2020, no earwigs have been reported from Arkansas, although scientists assume that four species reported from the adjoining state of Oklahoma occur in Arkansas, including the invasive European earwig.
Previously, there was general accord that the order comprised two major lineages: (1) the paraphyletic Protodermaptera (lower earwigs) and (2) the monophyletic Epidermaptera (higher earwigs), which are nested within the former. A phylogenomic study published in 2020 overturned these relationships by placing monophyletic Protodermaptera within paraphyletic Epidermaptera. Within Dermaptera, the family Apachyidae form the sister group to the remaining earwigs, which might imply that social behavior is not part of the ground plan of earwigs. Additional results from the study confirmed the monophyly of Eudermaptera within Epidermaptera and the paraphyly of several traditional families. The monophyly of Protodermaptera is reinforced by molecular and morphological evidence, although the exact position of the family Karschiellidae, which was not included in their molecular dataset, cannot be determined.
The fossil record of the Dermaptera begins in the Late to Early Triassic period, about 208 million years ago, in Australia and England; it comprises about seventy specimens in the extinct suborders Archidermaptera or Eodermaptera. Some of the morphological traits that belong to modern earwigs are not found in these early fossils, but adults possessed five-segmented tarsi (the final segment of the leg), well-developed ovipositors, veined tegmina (forewings), and long segmented cerci. Interestingly, the pincers would not have been curled or used as they are now. In 2020, a new species belonging to the extinct subfamily Astreptolabidinae (family Pygidicranidae) was described from mid-Cretaceous amber of Myanmar.
Throughout the history of systematic entomology, the relationships among these groups of earwigs have been difficult to resolve. In particular, nearly every other polyneopteran insect order has been considered as a sister group to Dermaptera. Many orders of insects have been theorized to be closely related to earwigs, though the icebugs of Notoptera—a family of extremophile (psychrophile) and wingless insects that live in the cold on top of mountains and the edges of glaciers—are most likely candidates. Insect taxonomists have previously assumed Dermaptera to be part of Polyneoptera, a monophyletic clade including roaches (Blattodea); stoneflies (Plecoptera); stick- and leaf-insects (Phasmatodea); and grasshoppers, crickets, and allies (Orthoptera), among others. Recent phylogenomic studies provided strong support for a sister-group relationship between Dermaptera and ground lice (Zoraptera).
Earwigs are abundant and can be found throughout Eurasia and the Americas. They inhabit tight crevices in fields, gardens, and woodlands. The common earwig (F. auricularia) was introduced from Europe into North America in 1907; it is common in the southern and southwestern parts of the United States. The only native species of earwig found in the northern United States is the spine-tailed earwig, Doru aculeatum, found as far north as southern Ontario, Canada, where it hides in the leaf axils of emerging plants in wetlands. However, other families can be found in North America, including Anisolabididae, Forficulidae (genera Doru and Forficula), Labiduridae, and Spongiphoridae.
Earwigs possess distinctive cerci, a pair of forceps-like pincers on their abdomen; males generally have more-curved pincers than females. They use these structures in various ways: (1) to capture and hold prey, (2) in copulation, and (3) to defend themselves. Species within Forficulina are free-living, have functional wings, and are not parasites. Their cerci are unsegmented and modified into large, forceps-like structures. Biting-type mouthparts are also present. The antennae of earwigs are thread-like and contain at least ten segments. Their abdomen is flexible and muscular. Earwigs have a unique way of folding their wings, with their short and leather-like membranous (very thin) hind wings under the short tegmina; they rarely use their forewings. The hindwing expands like a fan, radiating from one point folded under the forewing. Species in the former suborders Arixeniina and Hemimerina are wingless and blind with filiform segmented cerci. Even though most earwigs have wings and are capable of flight, they are rarely use their wings for flying.
Most earwigs have a flattened and elongated body, with specimens generally ranging in length from seven to 50 mm (0.28–1.97 in.). The Australian giant earwig (Titanolabis colossea) is the largest extant species at approximately 50 mm (2.0 in.) long, whereas the possibly extinct Saint Helena earwig (Labidura herculeana) reached 78 mm (3.1 in.).
Two families (Arixeniidae and Hemimeridae) of earwigs are tiny ectoparasites on Malay Peninsula bats and giant murid rats of sub-Saharan Africa, respectively; they lack typical pincers but possess specialized mouthparts for scraping fungus and dead skin off their host. Adaptations as external parasites (epizoic lifestyle) also include short broadened legs with grooves that allow them to cling to the host, loss of wings and eyes, and straight narrow cerci. In the Arixeniina, species of the genus Arixenia are normally found deep in the skin folds and gular pouch of Malaysian hairless bulldog bats (Cheiromeles torquatus), apparently feeding on bats’ body or glandular secretions. On the other hand, species in the genus Xeniaria (still of the suborder Arixeniina) are believed to feed on the guano and possibly the guanophilous arthropods in the bat’s roost, where it has been found. Hemimerina includes Araeomerus found in the nest of long-tailed pouch rats (Beamys), and Hemimerus found on giant Cricetomys rats.
Concerning reproduction, earwig mating takes place in the fall, and both sexes can be found together in the autumn and winter. Males possess a paired penis, and sperm is deposited in the female reproductive tract, where it may remain for months before the eggs are fertilized. From midwinter to early spring, the male will disperse on his own or will be driven out by the female. Afterward, the female will lay twenty to eighty eggs in two days. When first laid, the eggs are pearly white or cream-colored and oval-shaped, but immediately before hatching (in about seven days) they become kidney-shaped and brown. Each egg is approximately 1 mm (0.04 in.) tall and 0.8 mm (0.03 in.) wide. Some earwigs, especially those that are parasitic, give birth to live young (viviparous), with the young having been fed by a sort of placenta.
Many species of earwigs exhibit maternal care, which is rare among non-social insects. Females might care for their eggs, and even after nymphs emerge from the eggs, females will continue to guard their offspring until their second molt. As the nymphs molt, sexual dimorphism such as differences in pincer shapes begins to be evident. The male’s forceps will develop curved, whereas the females’ forceps remain straight. Earwigs are hemimetabolous, meaning they go through three major developmental stages to include egg, nymph, and adult, each developing through a series of four to six molts. The developmental stages of earwigs between molts are called instars. Earwigs live for about a year from hatching. The male and female will dwell in a chamber in debris, in crevices, or in soil 2.5 mm (1.0 in.) deep.
Most earwigs are nocturnal and inhabit small crevices, living in small amounts of debris such as bark and fallen logs. They often hide in small, moist crevices during the day and are active at night, feeding on a wide variety of insects and plants. They can usually be seen on household walls and ceilings. Interaction with earwigs at this time results in a defensive free-fall to the ground followed by a scramble to a nearby cleft or crevice. During the summer, they can be found around damp areas such as near sinks and in bathrooms. Earwigs tend to gather in shady cracks or openings or anywhere that they can remain concealed during daylight. Picnic tables, compost and waste bins, patios, lawn furniture, window frames, or anything with minute spaces (even artichoke blossoms) can potentially harbor them. Damage to foliage, flowers, and various crops is commonly blamed on earwigs, especially F. auricularia. Species on the island of Hawaii and in South Africa have been found to be blind and living in caves.
Earwigs are regularly preyed upon by birds, and like many other insect species they are prey for insectivorous mammals, amphibians, lizards, centipedes, assassin bugs, and spiders. European naturalists have observed bats preying upon earwigs. Their primary insect predators are parasitic species of Tachinidae, or tachinid flies, whose larvae are endoparasites. One species of tachinid fly, Triarthria setipennis, has been demonstrated to be successful as a biological control of earwigs for almost a century. Another tachinid fly and parasite of earwigs, Ocytata pallipes, has shown promise as a biological control agent as well. The common predatory wasp, the yellow jacket (Vespula maculifrons), preys upon earwigs when abundant. A small species of roundworm, Mermis nigrescens, is known to occasionally parasitize earwigs that have consumed roundworm eggs with plant matter. At least twenty-six species of parasitic fungus from the order Laboulbeniales have been found on earwigs. The eggs and nymphs are also cannibalized by other earwigs. A species of tyroglyphoid mite, Histiostoma polypore (Histiostomatidae, Astigmata), is observed on common earwigs, sometimes in great densities; however, this mite only feeds on earwig cadavers and not its live earwig transportation.
For protection from predators, the lined earwig, Doru taeniatum of North, Central, and South America can squirt foul-smelling yellow liquid in the form of jets from scent glands on the dorsal side of the third and fourth abdominal segment. It aims the discharges by revolving the abdomen, a maneuver that enables it simultaneously to use its pincers in defense.
Earwigs are mostly scavengers, but some are omnivorous or predatory. Food typically consists of a wide array of living and dead plant and animal matter. The common earwig is an omnivore, eating plants and ripe fruit (peaches, plums, grapes) as well as actively hunting arthropods. To a large extent, this species is also a scavenger, feeding on decaying plant and animal matter if given the chance. There is an argument about whether earwigs are harmful or beneficial to crops, as they eat both the foliage and the insects eating such foliage, such as aphids, though it would take a large population to do considerable damage. The common earwig eats a wide variety of plants, and also a wide variety of foliage, including the leaves and petals. They have been known to cause economic losses in fruit and vegetable crops. Some examples are the flowers, hops, red raspberries, and corn crops (corn silk) in Germany, and in the south of France, earwigs have been observed feeding on peaches and apricots. Other observed prey include plant lice, but also large insects such as bluebottle flies (Diptera) and woolly aphids (Hemiptera).
Earwigs are fairly abundant and are found in many areas around the world. There is no indication that they transmit diseases to humans or other animals. Their pincers are commonly assumed to be dangerous, but in truth, even the curved pincers of males cause little or no harm to humans. Earwigs have been rarely known to crawl into the ears of humans, but they do not lay eggs inside the human body.
In Arkansas, there is no list of species present in the state. It is assumed that four species reported from the adjoining state of Oklahoma occur in Arkansas, including the invasive European earwig. Research is sorely needed on this group of insects in the state.
For additional information:
Arnold, Don C., and W. A. Drew. “Earwigs (Dermaptera) of Oklahoma.” Proceedings of the Oklahoma Academy of Science 59 (1979): 115‒116.
Colgan, D., G. Cassis, and E. Beacham. “Setting the Molecular Phylogenetic Framework for the Dermaptera.” Insect Systematics and Evolution 34 (2003): 65–79.
Engel, M. S. “The Earwigs of Kansas, with a Key to Genera North of Mexico (Insecta: Dermaptera).” Transactions of the Kansas Academy of Science 106 (2009): 3–4.
Giles, E. “The Comparative External Morphology and Affinities of the Dermaptera.” Ecological Entomology 115 (1963): 95–164.
Haas, Fabian. “The Evolution of Wing Folding and Flight in the Dermaptera (Insecta).” Acta Zoologica Cracoviensia 46 (2003): 67–72.
Hoffman, Kevin M. “Earwigs (Dermaptera) of South Carolina, with a Key to the Eastern North American Species and a Checklist of the North American Fauna.” Proceedings of the Entomological Society of Washington 89 (1987): 1‒14.
Jarvis, Karl J., Fabain Haas, and Michael F. Whiting. “A Phylogeny of Earwigs (Insecta: Dermaptera) Based on Molecular and Morphological Evidence: Reconsidering the Classification of Dermaptera.” Systematic Entomology 30 (2004): 1–12.
Kamimura, Y. “In Search of the Origin of Twin Penises: Molecular Phylogeny of Earwigs (Dermaptera: Forficulina) Based on Mitochondrial and Nuclear Ribosomal RNA Genes.” Annals of the Entomological Society of America 97 (2004): 903–912.
Kurczewski, Frank E. “Vespula maculifrons (Hymenoptera: Vespidae) Preying on the European Earwig Forficula auricularia.” Journal of the New York Entomological Society 76 (1968): 84‒86.
Mao, Yue, Michael S. Engel, Ren Dong, and Taiping Gao. “A New Species of Astreptolabis in Mid-Cretaceous Amber from Northern Myanmar, With the Discovery of the First Male of Astreptolabidinae (Dermaptera).” Zookeys 911 (2020): 101‒112.
Sakai, S. “A New Proposed Classification of the Dermaptera with Special Reference to the Check List of the Dermaptera of the World.” Bulletin of Daito Bunka University 20 (1982): 1‒108.
Staerkle, M., and M. Koelliker. “Maternal Food Regurgitation to Nymphs in Earwigs (Forficula auricularia).” Ethology 114 (2008): 844–850.
Suzuki, S., M, Kitamura, and K. Matsubayashi. “Matriphagy in the Hump Earwig, Anechura harmandi (Dermaptera: Forficulidae), Increases the Survival Rates of the Offspring.” Journal of Ethology 23 (2005): 211–213.
Weiss, Michael J., and Garrick McDonald. “European Earwig, Forficula auriculari L. (Dermaptera: Forficulidae), as a Predator of the Redlegged Earth Mite, Halotydeus destructor (Tucker) (Acarina: Penthaleidae).” Australian Journal of Entomology 37 (2): 183–185.
Wipfler, Benjamin, Ward Koehler, Paul B. Frandsen, Sabrina Simon, et al. “Phylogenomics Changes Our Understanding About Earwig Evolution.” Systematic Entomology (2020): DOI: 10.111.syen.12420.
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology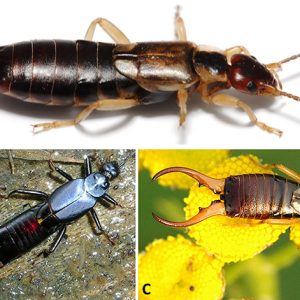 Earwigs
Earwigs 




Comments
No comments on this entry yet.