calsfoundation@cals.org
Chytrid Fungus
Chytrid (pronounced “kit-rid”) fungus belongs to the Kingdom Fungi, Phylum Chytridiomycota, Class Chytridiomycetes, and Order Rhizophydiales, a division of zoosporic organisms. The fungus causes the disease chytridiomycosis, originally generated by Batrachochytrium dendrobatidis (Bd). An amphibian chytrid (discovered in 1998)—which causes an infection of the skin, with an affinity to frogs and toads—is present on every amphibian-inhabited continent. Further, broad lineage and genotypic variation have been found in B. dendrobatidis. The disease has resulted in a serious decline and extinction of more than 200 species of amphibians worldwide and poses the greatest threat to biodiversity of any known disease. By 2021, the fungus had pushed the regression of at least 501 amphibian species, or about one out of every 16 (6.3 percent) described species. In Arkansas, amphibians affected include the Ozark hellbender and the Blanchard’s cricket frog.
A similar species, B. salamandrivorans (Bsal), originally discovered in 2013, also causes chytridiomycosis but primarily occurs in caudate (urodelan) amphibians (salamanders). It was initially discovered in captive and native fire salamanders (Salamandra salamandra) in Belgium and the Netherlands, where it led to significant declines in salamander populations. Since then, Bsal has also been noticed in captive salamanders in the United Kingdom and Germany.
Research suggests that Bsal is probably endemic to Asia and may have been introduced into Europe through the global pet trade. Between 2010 and 2014, over 750,000 salamanders were imported into the United States alone, creating an elevated probability that Bsal could ultimately be introduced into the country. Researchers suggest that introduction into the United States appears to be chief for the Pacific coast, southern Appalachian Mountains, and mid-Atlantic regions. By 2021, however, Bsal had not been confirmed in North America. Unlike B. dendrobatidis, B. salamandrivorans produces nonmotile, cell‐walled zoospores that can persist in environmental substrates for longer durations.
Batrachochytrium spp. are the only chytrids that are parasitic on vertebrates (specifically anuran and caudate amphibians). Interestingly, the fungus has also been reported from caecilian amphibians from the Republic of Cameroon and the United Republic of Tanzania. To date, this fungus has not been reported to infect other vertebrates such as reptiles, birds, or mammals, but there are makings for non-amphibian hosts, including crayfish, fish, and waterfowl. Indeed, there are approximately 1,000 different chytrid species that live exclusively in aquatic or moist environments. The chytrids are among the most primitive types of fungi, and the bulk are saprobes that feed on dead and rotting organic matter. Other chytrids are parasites that live on plants and invertebrates.
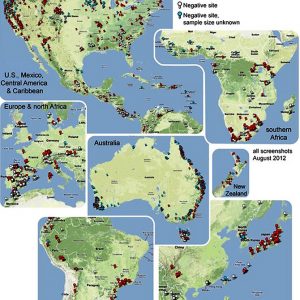
Bd was first noted when the golden toad (Incilius periglenes, formerly Bufo periglenes) and about fifty percent of the frog species at the Monteverde Cloud Forest Biological Reserve of Costa Rica disappeared in 1987. Since then, the fatal disease has been expanding eastward through the Central American highlands and, likely from a separate introduction, across a large portion of the Andes Mountains. It is also actively dispersing in central and western North America, as well as Australia, the Caribbean, and Europe. However, although Bd is found in Africa, Asia, and eastern North America, it does not seem to be spreading in these locations. Bd is conspicuously absent from Borneo, Madagascar, and New Guinea, where it could be devastating on rare amphibians. There is historical and genetic evidence that Bd has been present for a long time in Africa, Japan, and the eastern part of North America, but Eastern Asia has been proposed as the possible site of origin.
These chytrid fungi are very important pathogens because they appear to be capable of infecting most of the world’s approximately 6,000 amphibian species. Many infected taxa develop chytridiomycosis, which is linked to steep population declines and species extinctions. Amphibian population declines can occur very rapidly due to chytridiomycosis, sometimes in just a few weeks, and inexplicably eliminate rare or endangered, unique, and endemic species. Because of these characteristics, especially rapid progression of population declines and loss of important amphibian species, it is crucial to organize conservation efforts to help sustain amphibian species.
The disease occurs when an amphibian is infected with a significant amount of the Bd fungus inside the cells of the outer skin layers that contain large amounts of the waterproofing protein keratin. Using light microscopy to view epidermal biopsies, hosts with chytridiomycosis possess skin that becomes very thick, a term pathologists refer to as “hyperkeratosis and/or hyperplasia.” These changes become harmful over time and potentially deadly because amphibians consume water and absorb important electrolytes (like sodium and potassium) through their skin. Thus, the infection leads to abnormal electrolyte levels as a result of this Bd-damaged skin and may damage the host’s heart, resulting in death. Salamanders, like those in the family Plethodontidae (lungless salamanders), use their skin to breathe, and skin changes due to Bsal could interfere with their usual respiration, causing asphyxia.
Clinical signs of an amphibian that is suffering from chytridiomycosis can involve a multitude of symptoms. Some of the most common signs are: (1) reddened or otherwise discolored skin and excessive shedding (sloughing) of skin, (2) abnormal stances such as a preference for keeping the skin of the belly away from the ground, (3) anomalous behaviors such as a nocturnal species that suddenly becomes diurnal, and (4) seizures. However, many of these are non-specific signs, and several different amphibian diseases have characteristics that overlap with those of chytridiomycosis. In addition, other cases of chytridiomycosis will not reveal any of these signs, and some amphibians will simply be found already dead from the disease. For these reasons, it is impossible to diagnose chytridiomycosis macroscopically and specific laboratory tests are required.
The Bd infection spreads and is transmitted by a motile asexual reproductive fungal spore called a zoospore or swarm spore. Unlike Bd, Bsal produces nonmotile, cell‐walled zoospores that can persist in environmental substrates for longer durations. This stage in Bd has a distinctive appearance, with a single flagellum that helps the spore move about through aquatic environments. They require adequate dampness and cool temperatures and are capable of persisting in moist environments for several months but do not tolerate arid conditions for more than a few hours. Therefore, the most common and successful ways that Bd zoospores spread from site to site are in aqueous environs, on damp materials (including soil or equipment), or on the skin of infected amphibians. In fact, the most common way that Bd infection spreads between amphibians is from direct interaction of an infected individual with an uninfected specimen, especially during territoriality or reproductive activities.
Guidelines to reduce the transmission of Bd in captive environments are available. For example, in captive amphibians, it is entirely possible to house those infected with Bd in enclosures (aquarium or terrarium) side by side with uninfected amphibians in different enclosures and not transmit the infection, as long as animals, their water, wet materials, and cleaning tools are not shared between the enclosures.
In their native environment, it has been suggested that Bd can be transmitted from people’s shoes/boots or equipment or on birds and invertebrates that fly between watersheds. Therefore, it is important that biologists and pet keepers take precautions to clean and disinfect their footwear and equipment before moving from one location that has amphibians to another location in order to minimize the risk of spreading Bd. Because many amphibians that are infected with Bd are resistant to the disease chytridiomycosis, they can appear to be healthy but are still capable of spreading Bd from one location to another. This is very important because these animals may act as a reservoir for transmitting Bd infection to other amphibians as part of natural migration between different watersheds. Amphibians can also move Bd to new locations as the result of trade in amphibians or potentially by the release of captive amphibians into the wild.
The disease is diagnosed by obtaining a skin biopsy from a suspected host and examining the sample under light microscopy in search of the characteristic Bd organism. In addition, a non-invasive technique using sterile cotton swabs of the skin can be used and the sample analyzed by a molecular technique, the polymerase chain reaction (PCR). This technique can detect very small amounts of Bd DNA in a sample, and for this reason is the gold standard for detecting animals that carry Bd infection and to survey wild and captive amphibian populations for the presence of Bd. However, one major disadvantage of PCR is that it cannot distinguish between amphibians that are sick with chytridiomycosis and amphibians that are carriers of Bd, because both hosts will test positive.
Treatment of captive amphibians that have chytridiomycosis is often successful with antifungal medications (like itraconazole or Sporanox) and by disinfection of contaminated enclosures. Bathing infected specimens using a simple protocol of thirty-minute immersions in a 0.01 percent solution of itraconazole over a period of eleven days has been successful. Other potential treatment methods include the use of elevated body temperature and the antibiotic chloramphenicol. Treatment is not always effective, however, and not all hosts tolerate treatment well. There are no good methods for the treatment of wild amphibians in their native environment. It is very difficult or impossible to get enough of the antifungal medications into the environment to be able to successfully rid infected frogs of Bd. Another promising area of research is introducing symbiotic bacteria that inhibit the growth of Bd into wild amphibian populations. So far, there is no evidence that a vaccine for chytridiomycosis could be effective for controlling the disease in wild populations.
Interestingly, not all amphibian species that are infected with Bd fall ill, become moribund, or die. Any resistant species are a major concern because they function as carriers of Bd. That is, they are capable of transporting the fungus to new locales and exposing naïve populations of other amphibians that are susceptible or more likely to become ill with lethal chytridiomycosis.
Some of the mechanisms that could explain amphibian species resistance to chytridiomycosis are, first, the presence on their skin of specific types of symbiotic bacteria that depress the growth of the fungus and, second, production by the poison glands in amphibian skin of chemicals referred to as antimicrobial peptides that discourage the growth of Bd. In addition, populations that normally have large numbers of these bacteria in the skin might be more resistant to developing chytridiomycosis; specific types, combinations, or amounts of antimicrobial peptides might help some species to be more resistant to the disease; and some amphibian species or populations may have genetic resistance to the development of chytridiomycosis by mechanisms that are not yet fully understood. Some other potential explanations focus on environmental differences between populations, such as temperature, humidity, or water flow patterns. For example, some of the most important amphibian population declines associated with chytridiomycosis have occurred at locations at higher elevations that have a cooler temperature range (less than 25° C or 77° F) most optimal for the growth of the fungus. In addition, there are differences in virulence between different strains of Bd. One type of Bd that is highly virulent easily makes amphibians sick, but another type has low virulence and makes fewer animals sick or results in minor pathogenic disease. Although these reasons seem plausible, there is not a single explanation for why an amphibian population dies or does not die due to chytridiomycosis, and, as in most cases, multiple factors are probably at work.
In terms of recovery from infections, some amphibian populations obviously experience overwhelming mass mortality events due to chytridiomycosis in which most of the population succumbs to the disease; however, a small number of hosts persist in the population. It is unknown if these persistent populations might eventually recover and regain the numbers of individuals they had prior to the infection or if these populations will remain small or even ultimately disappear. Recent research has revealed that a critical factor in determining if chytridiomycosis will cause extinction of an amphibian population is if the level of intensity of the infection with Bd crosses a certain threshold. What is interesting about the “persistent” populations is that the remaining animals are still infected with Bd, but at a lower or less lethal intensity. Understanding how individuals and persistent populations can maintain low-intensity infections with Bd could lead to methods for controlling the disease in native populations.
It has become clear that worldwide trade in amphibians for use as laboratory animals, as captive pets, for display in zoos or collections, or as food is responsible for spread of Bd to locations where it was not previously found in amphibians. Many amphibian collections unknowingly have Bd-infected frogs. Therefore, international regulations have been set under the World Organization for Animal Health that require amphibians to be negative for Bd infection before they are shipped overseas.
Several suggestions for establishing Bd-free amphibians are to: (1) quarantine new amphibians before they enter an established amphibian collection; new individuals are kept separate from the established collection for a period of time (usually 60 to 90 days) to allow for observation for signs of disease and to perform laboratory testing for Bd, (2) test or treat animals for Bd infection during the quarantine period, (3) perform surveillance for Bd infection in an amphibian collection; this is accomplished by regular necropsies (autopsies) of animals that die and by PCR testing of captive specimens, (4) develop specific pathogen-free amphibian populations that are known to be negative for Bd infection; if all captive raised amphibians can be certified as Bd-free it simplifies quarantine and amphibian shipment practices, and (5) practice good hygiene and barrier management between organism rooms and displays, such as utilizing separate equipment and disposable sterile gloves between enclosures, and disposing of solid wastes and waste water sensibly.
In Arkansas, ten to twenty percent of endangered Ozark hellbenders (Cryptobranchus alleganiensis bishop) sampled from the Eleven Point River watershed in Randolph County were reported to harbor Bd. In another study, Bd was found in Blanchard’s cricket frogs (Acris blanchardi) from Wapanocca National Wildlife Refuge in Turrell (Crittenden County). However, no frog exhibited clinical signs of Bd infection (e.g., lethargy, skin sloughing), indicating that Bd in this population of A. blanchardi may persist in populations without succumbing to Bd-induced mortality. In a third study, frogs collected from the Bald Knob National Wildlife Refuge in White County and Felsenthal National Wildlife Refuge in Union County, including A. blanchardi from the former and southern leopard frogs (Rana sphenocephala) from the latter, harbored Bd. Because Arkansas supports about sixty species and subspecies of amphibians, much more work needs to be done in the state to survey additional populations of amphibians.
In terms of conservation efforts, the International Union of Conservation of Nature (IUCN) has called amphibian chytridiomycosis “the worst infectious disease ever recorded among vertebrates in terms of the number of species impacted and its propensity to drive them to extinction.” Recovery of wild populations informs current disease management strategies and provides hope for proactive policies and research directed toward disease control and amphibian conservation. Batrachochytrium‐induced chytridiomycosis threatens native amphibians globally, and continued research efforts and improvement of novel policies at both a national and international scale are sorely needed. In Australia, researchers discovered that treefrogs in Queensland survived in higher numbers near granite boulders and hypothesized that the rock created a warmer microhabitat, given the absorption of heat during the day, and thus protected the frogs from the fungus. Conservation biologist Anthony Waddle and others published a paper in Nature in 2024 on the utility of hotspot shelters in stimulating resistance to the fungal infection; they have since developed small greenhouses that serve as “frog saunas” for the treatment of local frogs.
For additional information:
Annis, S. L., F. P. Dastoor, H. Ziel, Peter Daszak, and J. E. Longcore. “A DNA-Based Assay Identifies Batrachochytrium dendrobatidis in Amphibians.” Journal of Wildlife Diseases 40 (2004): 420–428.
Antwis, R. E., and X. A. Harrison. “Probiotic Consortia are Not Uniformly Effective Against Different Amphibian Chytrid Pathogen Isolates.” Molecular Ecology 27 (2018): 577–589.
Bauer, K. L., J. C. Steeil, T. F. Walsh, M. J. Evans, B. Klocke, B. Gratwicke, J. L. Siegal-Willott, and D. L. Neiffer. “Batrachochytrium dendrobatidis in a Captive Collection of Green Salamanders (Aneides aeneus), Long-Tailed Salamanders (Eurycea longicauda), and Two-Lined salamanders (Eurycea bislineata).” Journal of Zoo and Wildlife Medicine 49 (2018): 454–459.
Berger, L., R. Speare, Peter Dazsak, D. E. Green, A. A. Cunningham, C. L. Goggin, R. Slocombe, M. A. Ragan, A. D. Hyatt, K. R. McDonald, H. B. Hines, Karen R. Lips, G. Marantelli, and H. Parkes. “Chytridiomycosis Causes Amphibian Mortality Associated with Population Declines in the Rain Forests of Australia and Central America.” Proceedings of the National Academy of Sciences USA 95 (1998): 9031‒9036.
Blaustein, Andrew R., J. M. Romansic, E. A. Scheessele, et al. Interspecific Variation in Susceptibility of Frog Tadpoles to the Pathogenic Fungus Batrachochytrium dendrobatidis. Conservation Biology 19 (2005): 1460–1468.
Blaustein, Andrew R., S. S. Gervasi, P. T. J. Johnson, J. T. Hoverman, L. K. Belden, P. W. Bradley, and G. Y. Xie. “Ecophysiology Meets Conservation: Understanding the Role of Disease in Amphibian Population Declines.” Philosophical Transactions of the Royal Society B 367 (2012): 1688–1707.
Bosch, J., E. Sanchez-Tome, A. Fernandez-Loras, J. A. Oliver, M. C. Fisher, and T. W. J. Garner. “Successful Elimination of a Lethal Wildlife Infectious Disease in Nature.” Biological Letters 11 (2015): 20150874.
Boyle, D. G., D. B. Boyle, V. Olsen, J. A. T. Morgan, and A. D. Hyatt. “Rapid Quantitative Detection of Chytridiomycosis (Batrachochytrium dendrobatidis) in Amphibian Samples Using Real-Time Taqman PCR Assay.” Diseases of Aquatic Organisms 60 (2004): 133‒139.
Daszak, Peter, A. Streiby, A. A. Cunningham, J. E. Longcore, C. C. Brown, and D. Porter. “Experimental Evidence that the Bullfrog (Rana catesbeiana) is a Potential Carrier of Chytridiomycosis, an Emerging Fungal Disease of Amphibians.” Herpetological Journal 14 (2004): 201–207.
Fisher, M. C., T. W. J. Garner, and S. F. Walker. “Global Emergence of Batrachochytrium dendrobatidis and Amphibian Chytridiomycosis in Space, Time, and Host.” Annual Review of Microbiology 63 (2009): 291–310.
Garner, T. W. J., M. Perkins, P. Govindarajulu, D. Seglie, S. J. Walker, A. A. Cunningham, and M. C. Fisher. “The Emerging Amphibian Pathogen Batrachochytrium dendrobatidis Globally Infects Introduced Populations of the North American Bullfrog, Rana catesbeiana.” Biological Letters 2 (2006): 455‒459.
Gower, D. J., T. Doherty-Bone, S. P. Loader, M. Wilkinson, M. T. Kouete, B. Tapley, et al. “Batrachochytrium dendrobatidis Infection and Lethal Chytridiomycosis in Caecilian Amphibians (Gymnophiona).” EcoHealth 10 (2013): 173–183.
Hanlon, S. M., D. Smith, J. L. Gerby, E. Berg, W. Peterson, M. J. Parris, and J. E. Moore. “Occurrence of Batrachochytrium dendrobatidis in Wapanocca National Wildlife Refuge, Arkansas, USA.” Herpetological Review 45 (2104): 31‒32.
Harris, R. N., A. Lauer, M. A. Simon, J. L. Banning, and R. A. Alford. “Addition of Antifungal Skin Bacteria to Salamanders Ameliorates the Effects of Chytridiomycosis.” Diseases of Aquatic Organisms 83 (2009): 11‒16.
Johnson, M. L., and R. Speare. “Possible Modes of Dissemination of the Amphibian Chytrid Batrachochytrium dendrobatidis in the Environment” Diseases of Aquatic Organisms 65 (2005): 181‒186.
———. “Survival of Batrachochytrium dendrobatidis in Water: Quarantine and Disease Control Implications.” Emerging Infectious Diseases 9 (2003): 922‒925.
Lips, Karen R. “Overview of Chytrid Emergence and Impacts on Amphibians.” Philosophical Transactions of the Royal Society of London B Biological Science 371 (2016): 20150465.
Lips, Karen R., F. Brem, R. Brenes, J. D. Reeve, R.A. Alford, J. Voyles, C. Carey, L. Livo, J. E. Longcore, A. P. Pessier, and D. K. Nichols. “Batrachochytrium dendrobatidis Gen. Et Sp. Nov., a Chytrid Pathogenic to Amphibians.” Mycologia 91 (1999): 219‒227.
Longcore JR, J. E. Longcore, A. P. Pessier, and W. A. Halteman. “Chytridiomycosis Widespread in Anurans of Northeastern United States.” Journal of Wildlife Management 71 (2007): 435– 444.
Martel An, Annemarieke Spitzen-van der Sluijs, Mark Blooi, Wim Bert, Richard Ducatelle, Matthew C. Fisher, Antonius Woeltjes, Wilbert Bosman, Koen Chiers, Franky Bossuyt, and Frank Pasmans. “Batrachochytrium salamandrivorans Sp. Nov. Causes Lethal Chytridiomycosis in Amphibians.” Proceedings of the National Academy of Science USA 110 (2013): 15325‒15329.
Murray, K. A., L. F. Skerratt, R. Speare, and H. McCallum. “Impact and Dynamics of Disease in Species Threatened by the Amphibian Chytrid Fungus, Batrachochytrium dendrobatidis.” Conservation Biology 23 (2009): 1242‒1252.
O’Hanlon, S. J., Adrien Rieux, Rhys A. Farrer, Gonçalo M. Rosa, Bruce Waldman, Arnaud Bataille, Tiffany A. Kosch, Kris A. Murray, et al. “Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines.” Science 360 (2018): 621‒627.
Ouellet, M., I. Mikaelian, Bd Pauli, J. Rodrigue, and D. M. Green. “Historical Evidence of Widespread Chytrid Infection in North American Amphibian Populations.” Conservation Biology 19 (2005): 1431–1440.
Pessier, A. P., and J. P. Collins. “Emerging Infectious Disease and the Loss of Biodiversity in a Neotropical Amphibian Community.” Proceedings of the National Academy of Sciences USA 103 (2006): 3165‒3170.
Pounds, J. Alan, M. R. Bustamante, L. A. Coloma, J. A. Consuegra, et al. “Widespread Amphibian Extinctions from Epidemic Disease Driven by Global Warming.” Nature 439 (2006): 161–167.
Rachowicz, L. J., J. Hero, R. A. Alford, J. W. Taylor, J. A. T. Morgan, V. T. Vrendenberg, J. P. Collins, and C. J. Briggs. “The Novel and Endemic Pathogen Hypotheses: Competing Explanations for the Origin of Emerging Infectious Diseases of Wildlife.” Conservation Biology 19 (2005): 1441‒1448.
Richgels, Katherine L. D., Robin E. Russell, Michael J. Adams, C. LeAnn White, and Evan H. Campbell Grant. “Spatial Variation in Risk and Consequence of Batrachochytrium salamandrivorans Introduction in the USA.” Royal Society of Open Science 3 (2016): 150616.
Rosenblum, E. B., J. Voyles, T. J. Poorten, and J. E. Stajich. “The Deadly Chytrid Fungus: A Story of an Emerging Pathogen.” PLoS Pathogens 6 (2010): e1000550.
Rothermel, Betsie B., Susan C. Walls, Joseph C. Mitchell, C. Kenneth Dodd, Jr., Lisa K. Irwin, David E. Green, Victoria M. Vazquez, James W. Petranka, and Dirk J. Stevenson. “Widespread Occurrence of the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis in the Southeastern USA.” Diseases of Aquatic Organisms 82 (2008): 3‒18.
Rowley, Jodi J. L., and Ross A. Alford. “Hot Bodies Protect Amphibians against Chytrid Infection in Nature.” Scientific Reports 3 (2013): 1‒4.
Scheele, Ben C., Frank Pasmans, Lee F. Skerratt, Lee Berger, An Martel, Wouter Beukem, et al. “Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity.” Science 363 (2019): 1459‒1463.
Schloegel, L. M., A. M. Picco, A. M. Kilpatrick, A. J. Davies, A. D. Hyatt, and Peter Daszak. “Magnitude of the US Trade in Amphibians and the Presence of Batrachochytrium dendrobatidis and Ranavirus Infection in Imported North American Bullfrogs (Rana catesbeiana).” Biological Conservation 142 (2009): 1420‒1426.
Skerratt, L. F., L. Berger, R. Speare, S. Cashins, K. R. McDonald, A. Phillott, H. Hines, and N. Kenyon. “Spread of Chytridiomycosis has Caused the Rapid Global Decline and Extinction of Frogs.” EcoHealth 4 (2007): 125‒134.
Soto-Azat, C., B. T. Clarke, J. C. Poynton, and A. C. Cunningham. “Widespread Historical Presence of Batrachochytrium dendrobatidis in African Pipid Frogs.” Diversity and Distributions 16 (2010): 126‒131.
Stice, M. J., and C. J. Briggs. “Immunization is Ineffective Against Preventing Infection and Mortality Due to the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis.” Journal of Wildlife Diseases 46 (2010): 70‒77.
Trauth, Stanley E., Henry W. Robison, and Michael V. Plummer. The Amphibians and Reptiles of Arkansas. Fayetteville: University of Arkansas Press, 2004.
Voyles, J., S. Young, L. Berger, C. Campbell, W. F. Voyles, A. Dinudom, D. Cook, R. Webb, R. A. Alford, L. F. Skerratt, and R. Speare. “Pathogenesis of Chytridiomycosis, a Cause of Catastrophic Amphibian Declines.” Science 326 (2009): 582‒585.
Weldon, Ché, Louis H. du Preez, Alex D. Hyatt, Reinhold Muller, and Rick Speare. “Origin of the Amphibian Chytrid Fungus.” Emerging Infectious Diseases 10 (2004): 2100‒2105.
Woodhams, Douglas C., Kelly L. Barnhart, Molly C. Bletz, Alberto J. Campos, Steven J. Ganem, Andreas Hertz, Brandon C. LaBumbard, Priya Nanjappa, and Amanda G. Tokash‐Peters. Batrachochytrium: Biology and Management of Amphibian Chytridiomycosis. Chidester: John Wiley & Sons Ltd., 2018.
Yap, T. A., M. S. Koo, R. F. Ambrose, and V. T. Vredenburg. “Introduced Bullfrog Facilitates Pathogen Invasion in the Western United States.” PLoS One 13 (2018): e0188384.
Chris T. McAllister
Eastern Oklahoma State College







Comments
No comments on this entry yet.