calsfoundation@cals.org
Odonates
aka: Dragonflies
aka: Damselflies
Odonates belong to the Phylum Arthropoda, Subclass Pterygota (winged insects), Class Insecta, and Order Odonata. The famous entomologist Johan Christian Fabricius (1745‒1808), who was the first to provide the name Odonata, apparently used that name because they have “teeth” on their mandibles. Odonates are considered a wide-ranging, primitive order of carnivorous aquatic insects. About 102 species have been documented in Arkansas.
One of the two extant suborders of the Odonata (the order to which dragonflies and damselflies belong) is the Epiprocta. This new classification was proposed relatively recently to accommodate the inclusion of the suborder Anisozygoptera. The latter taxon has been shown not to be a natural suborder but somewhat of a paraphyletic collection of lineages, so it has been united with the previous suborder that included the well-known dragonflies, the Anisoptera, into the Epiprocta. The old suborder Anisoptera has been proposed to become an infraorder within the Epiprocta, whereas the “anisozygopterans” included here form the infraorder Epiophlebioptera. Some, however, consider this reclassification based on molecular studies of the Zygoptera still preliminary and do not follow it pending confirmation by further studies.
Evolutionarily speaking, odonates are distantly related to the mayflies (Ephemeroptera), as both are placed in the ancient winged insect group, the Palaeoptera, which has only two extant orders (Ephemeroptera and Odonata). The Palaeoptera has been traditionally applied to those ancestral groups of winged insects that possess wings that cannot be folded back over the abdomen. Indeed, when at rest, their wings are either held straight out to the side of the body or vertically above the abdomen.
Ancestors of odonates date back more than 300 million years (Late Carboniferous Epoch) and predate the dinosaurs by about 100 million years, and the order contains four extinct suborders: (1) Protanisoptera of the Permian Period (299 to 251 million years ago); (2) Archizygoptera of the same period; (3) Triadophlebiomorpha of the Triassic Period (251 to 200 million years ago); and (4) Anisozygoptera of the Triassic-Cretaceous periods (251 to 65 million years ago).
Odonates are primitive aquatic insects that exhibit a number of ancestral traits that were probably present in the earliest flying insects, such as long tails and wings that do not fold flat over their abdomens. They are represented in the fossil record by giant dragonflies in the Paleozoic such as Meganeuropsis permiana from the Permian of North America, which had a wing span of 70 cm (28 in.) and a body length of 43 cm (17 in.), making it one of the largest insects ever known. These former giants were in the order Meganisoptera (griffinflies) related to odonates, but not part of the modern order Odonata stricto sensu. Today, the largest odonate is the giant Central American helicopter damselfly (Megaloprepus coerulatus, family Pseudostigmatidae) with a wing span of 19 cm (7.5 in.). The title of the longest living odonate goes to the South American helicopter damselfly (Mecistogaster linearis), with a body length of 14 cm (5.5 in.). The smallest living dragonfly is Nannophya pygmaea (family Libellulidae), which lives in eastern Asia and has a body length of only 1.5 cm (0.6 in.) and a wing span of only 2 cm (0.8 in.). The smallest damselflies and smallest odonates of all time are species of the genus Agriocnemis (Zygoptera: Coenagrionidae), which have a wing span of only 1.8 cm (0.7 in.).
There are approximately 6,000 species of odonates (dragonflies and damselflies) described worldwide. The dragonflies number about 3,000 species grouped in eight living families, while damselflies number just under 3,000 species contained within nineteen different families. Odonates are distributed around the world and are especially numerous and varied in the tropics, although they range north to the boreal forests of Siberia and North America. They are quite common in the Southern Hemisphere, except Antarctica. As of 2019, there are 468 species of Odonata within seven families of damselflies and eleven families of dragonflies known to occur in North America north of Mexico. There are thought to be about 102 species documented for Arkansas. Recently, the least clubtail has become a separate species from the major population and now has the common name interior least clubtail (Stylogomphus sigmastylus), and a new spiketail described in 2004 is called the Ouachita spiketail (Cordulegaster talaria). It is known from only three counties in Arkansas and one county in southeastern Oklahoma.
As of 2019, no recent single study of all odonates exists specifically for Arkansas; however, George L. Harp, professor emeritus at Arkansas State University, gained a reputation as the preeminent expert on the dragonflies of Arkansas. Harp collected odonates in most of the state’s seventy-five counties and published widely on them. Another odonate researcher from the state was John D. Rickett (1944‒2002) of the University of Arkansas at Little Rock. He and Harp provided a list of the ninety-one extant species of odonates known from the state.
Dragonflies are generally larger and perch with their wings held out to the sides, while damselflies have slender bodies and hold their wings over the body at rest. Dragonflies are often strikingly colored and are active primarily during the day, although there is at least one Arkansas species, the orange shadowdragon (Neurocordulia xanthosoma), that is active only at dawn and dusk.
Characteristically, adult odonates have large, rounded, dumbbell-shaped heads; three ocelli; a pair of short, inconspicuous setaceous antennae and compound eyes; and two pairs of large, transparent membranous wings with many longitudinal veins and cross veins that move independently. In dragonflies, these wings are held outstretched, whereas they are straight up when at rest over the slender body and ten-segmented abdomens of damselflies. Large wing muscles attach to the sloping thorax, which sets the legs forward where they can best catch insects in flight. The chewing mouthparts include simple but massive mandibles in the adults and are located on the underside of the head.
The two pairs of large wings found in odonates are strengthened by a complex network of veins. In most families there are specialized cells on the leading edge near the tip of each outer wing known as the pterostigma. This thickened patch is filled with hemolymph and is a colorful area bounded by veins. While the function is uncertain, it probably has an aerodynamic effect and perhaps a visual function. Odonates use their wings in a unique manner. Whereas other insects with four wings beat them synchronously, odonates can beat the fore and hind pairs independently. This allows three different modes of flight in which the pairs beat: (1) synchronously, as those of other insects; (2) alternately between the two sets; or (3) synchronously but out of phase with each other. Such variations allow odonates a series of aerobatic abilities that include hovering, backward flight, and turns of such tight radius that they are virtually midair pivots.
While adult damselflies and dragonflies are superficially similar, several obvious characteristics easily separate the two groups. Adult dragonflies are strong fliers with generally robust bodies, and they tend to hold their wings out, either straight to the side or downward when at rest. Damselflies tend to be more delicate and much less robust, and they hold their wings folded back over the abdomen when at rest. In addition, dragonflies possess large compound eyes that occupy most of the head region and either touch or nearly touch each other across the face. In damselflies, however, there is usually a gap between the eyes. These large, compound eyes in most dragonflies can consist of 30,000 ommatidia (separate light-sensing optical units). This is thought to provide some dragonflies with an almost 360-degree view of their habitat. This may help explain why it is so difficult to catch them.
Odonates have aquatic larvae called naiads or nymphs that are wingless and live in a variety of shallow freshwater habitats including lakes, ponds, marshes, streams, rivers, and even holes in trees. These drab or mottled benthic larval forms have short, stocky, well-camouflaged bodies that lack wings and possess heads that are less mobile than adults heads. The head region has smaller eyes and longer antennae, and mouthparts are modified with a labium with a unique prehensile organ for grasping prey. As with differences between their respective adult forms, damselfly nymphs differ morphologically from dragonfly nymphs. Dragonfly naiads are more robust or elongated and are gray, brown, or greenish in color. They tend to inhabit submerged vegetation or woody debris on the bottoms of ponds, shallow lake regions, streams, and rivers. Alternatively, damselfly nymphs are more slender and generally smaller, about 1.0 to 2.2 cm (0.4 to 0.9 in.) in length compared to dragonfly naiads, which are about 1.5 to 4.5 cm (0.6 to 1.8 in.) in length with three external, leaf-like tracheal gills at the tip of the abdomen.
Reproduction in odonates is accomplished by adults mating in the air. When the adult female enters the territory of a male, the resident male tries to mate with her. In some species mating is preceded by a courtship display during which the female accepts or rejects the male. If accepted, the male tries to guide the female to an egg-laying site in his territory. Generally, there is no prelude to mating and thus mating occurs immediately. Adult males have complex genitalia that differ from those in other insects. For example, males have accessory genitalia on the second and third abdominal sternites (ventral part of a shield), which include grasping cerci that hold the female and the secondary set of copulatory organs on the ninth segment of the abdomen in which sperm are held. Before actual mating can occur, the male dragonfly must charge his secondary copulatory apparatus with sperm from his primary copulatory apparatus. During actual mating, the male grasps the female with his abdominal claspers by the thorax or head and bends her abdomen so that her own genitalia can be grasped by the male’s copulatory organs containing sperm. This unusual reproductive position is known to entomologists as the “wheel position.” Copulation may take from several minutes to several hours depending on species. After mating occurs, males may stay in tandem with the female while her eggs are laid in water or on vegetation near water. The male may stay close to the female to guard her while she lays, while in those strongly territorial species, the male may be satisfied by continuing to expel other males from his territory, allowing the female to lay within the territory. As with other aquatic insects, odonates then hatch into pro-larvae and then go through a series of stages or instars (number varies with each species), molting nine to seventeen times, each time resulting in larger and more development before they metamorphose into adults. Larval development can range from three weeks to over eight years, depending on the species and habitat. Nymphs sometimes reach lengths of more than 5 cm (2 in.). Most temperate species spend their last winter in the final instar stage and emerge during the spring or summer. Emergence usually occurs after sunset if the temperatures are high enough, and they take flight just prior to sunrise.
Nymphal odonates are vicious predators that feed on all types of aquatic invertebrates and even small fishes. As adults, they feed upon other live flying insects, including other odonates (dragonflies eating damselflies). Dipterans (gnats, midges, and mosquitoes) make up the bulk of their diet, but they also prey on bees, beetles, butterflies, other flies, and moths. Larval odonates serve as food for crayfishes, fishes, birds, amphibians, and reptiles, while adults are fed upon by spiders, fishes, birds, and amphibians and reptiles.
Species of odonates have been referred to as “generalists” or “plastic” species because they tolerate a wide range within many environmental parameters. The nymphs of these generalists have the ability to survive in relatively low dissolved oxygen levels and, therefore, are of primary importance. They can inhabit a wide range of current speeds and are characteristically found in both lentic and lotic habitats. Examples of strongly lentic species include Anax longipes, Celithemis spp., Enallagma aspersum, E. traviatum, and Lestes disjunctus australis. Species in which habitat preferences are strongly lotic are Argia sedula, Basiaeschna janata, Enallagma exsulans, Hagenius brevistylus, Hetaerina americana, and Stylogomphus sp. Seeps and springs are also important habitats for dragonflies such as these species found in seeps: Argia bipunctulata, Cordulegaster obliqua, Tachopteryx thoreyi; and Argia plana (springwater dancer) found only at springs.
Males spend their time defending territories that are typically located near the margin of a body of water such as a pond, lake, or stream. These sites may be intensely defended, and an individual male may return to the same perch for many successive days as he continues to expel other male intruders. Interactions between rival males may be quite violent and occasionally result in injury or death. When a dragonfly perches on a leaf, twig, or another object, it often lifts its abdomen and points its tail upward. This is referred to as “obelisking,” and this behavior’s purpose is unknown.
Three common damselfly families in Arkansas with their common species are the following: Coenagrionidae (Amphiagrion spp., Argia fumipennis, A. moesta, A. sedula, Enallagma spp., and Ischnura posita); Lestidae (Lestes disjunctus and L. vigilax); and Calopterygidae (Calopteryx maculata and Hetaerina americana). Six common Arkansas dragonfly families with common species inhabiting the state are the following: Aeshnidae (Aeshna constricta, Basiaeschna janata, Boyeria vinosa, Epiaeschna heros, and Nasiaeschna pentacantha); Petaluridae (Tachopteryx thoreyi); Cordulegastridae (Cordulegaster maculata ); Cordullidae (Epitheca cynosura, E. princeps, Didymops transversa, Macromia illinoiensis, Neurocordulia molesta, and Somatocholora linearis); Gomphidae (Arigomphus lentulus, Dromogomphus spinosus, D. spoliatus, Gomphurus externus, G. graslinellus, Hagenius brevistylus, Ophiogomphus westfalli, Progomphus obscurus, Stylogomphus sigmastylus); and Libellulidae (Celithemis elisa, C. eponina, Dythemis velox, Erythemis simplicicoliis, Libellula cyanea, L. luctuosa , L. vibrans, Plathemis lydia, and Sympetrum ambiguum). One of the most common odonates in the state is the eastern pondhawk (Erythemis simplicicollis), which has been documented in all of Arkansas’s seventy-five counties.
Although there is not any endemic damselfly or dragonfly species in Arkansas, there are two dragonflies that are endemic to the uplands of the Interior Highlands (Ozark Plateau and Ouachita Mountains) including Westfall’s snaketail (Ophiogomphus westfalli) from Arkansas and Missouri, and Ozark emerald (Somatochlora ozarkensis) from Arkansas, Kansas, Missouri, and Oklahoma. Both are considered critically imperiled (S1) in Arkansas according to NatureServe.
For additional information:
Abbott, John C. Damselflies of Texas: A Field Guide. Austin: University of Texas Press, 2011.
———. Dragonflies and Damselflies of Texas and the South-Central United States: Texas, Louisiana, Arkansas, Oklahoma, and New Mexico. Princeton, NJ: Princeton University Press, 2005.
———. Dragonflies of Texas: A Field Guide. Austin: University of Texas Press, 2015.
Adams, C. C. “Odonata from Arkansas.” Entomological News 11 (1900): 621‒622.
Allen, Robert T. “Additions to the Known Endemic Flora and Fauna of Arkansas.” Proceedings of the Arkansas Academy of Science 42 (1988): 18–21. Online at https://scholarworks.uark.edu/jaas/vol42/iss1/8/ (accessed December 6, 2019).
———. “Insect Endemism in the Interior Highlands of North America.” Florida Entomologist 73 (1990): 539–569.
Bick, G. H. “Additional Dragonflies (Odonata) from Arkansas.” Southwestern Naturalist 4 (1959): 131‒133.
Boys, Wade Alexander. “Biogeography of Endemic Dragonflies of the Ozark-Ouachita Interior Highlands.” MS thesis, University of Arkansas, 2019. Online at https://scholarworks.uark.edu/etd/3245/ (accessed July 6, 2022).
Cather, Mary R., and George L. Harp. “The Aquatic Macroinvertebrates of an Ozark and a Deltaic Stream.” Proceedings of the Arkansas Academy of Science 29 (1975): 30‒35. Online at https://scholarworks.uark.edu/jaas/vol29/iss1/11/ (accessed December 6, 2019).
Chordas, Stephen W., III., George L. Harp, and G. W. Wolfe. “Aquatic Macroinvertebrates of the White River National Wildlife Refuge, Arkansas.” Proceedings of the Arkansas Academy of Science 50 (1996): 42‒51. Online at https://scholarworks.uark.edu/jaas/vol50/iss1/9/ (accessed December 6, 2019).
Cochran, Betty G., and George L. Harp. “Aquatic Macroinvertebrates of the St. Francis Sunken Lands in Northeast Arkansas.” Proceedings of the Arkansas Academy of Science 44 (1990): 24‒27. Online at https://scholarworks.uark.edu/jaas/vol44/iss1/8/ (accessed December 6, 2019).
Cook, C., and J. J. Daigle. “Ophiogomphus westfalli Spec. Nov. From the Ozark Region of Arkansas and Missouri, with a Key to the Ophiogomphus Species of Eastern North America (Anisoptera: Gomphidae).” Odonatologica 14 (1985): 89‒99.
Dunkle, S. W. “Critical Species of Odonata in North America.” International Journal of Odonatology 7: 149–162.
———. Dragonflies through Binoculars: A Field Guide to Dragonflies of North America. Oxford: Oxford University Press, 2000.
Harp, George L. “A Preliminary Report on the Zygoptera (Damselflies) of Arkansas.” Proceedings of the Arkansas Academy of Science 37 (1983): 87‒88. Online at https://scholarworks.uark.edu/jaas/vol37/iss1/30/ (accessed December 6, 2019).
Harp, George L., and Phoebe A. Harp. “Dragonflies (Odonata) of the Ouachita National Forest.” Journal of the Arkansas Academy of Science 57 (2003): 68‒75. Online at https://scholarworks.uark.edu/jaas/vol57/iss1/10/ (accessed December 6, 2019).
———. “Previously Unpublished Odonata Records for Arkansas, Kentucky, and Texas.” Notulae Odonatologicae 4 (1996): 127‒130.
Harp, George L., and John D. Rickett. “The Dragonflies (Anisoptera) of Arkansas.” Proceedings of the Arkansas Academy of Science 31 (1977): 50‒54. Online at https://scholarworks.uark.edu/jaas/vol31/iss1/17/ (accessed December 6, 2019).
———. “Further Distributional Records for Arkansas Anisoptera.” Proceedings of the Arkansas Academy of Science 39 (1985): 131‒135. Online at https://scholarworks.uark.edu/jaas/vol39/iss1/38/ (accessed December 6, 2019).
Harp, George L., and Henry W. Robison. “Aquatic Macroinvertebrates of the Strawberry River System in North-Central Arkansas.” Journal of the Arkansas Academy of Science 60 (2006): 46‒61. Online at https://scholarworks.uark.edu/jaas/vol60/iss1/9/ (accessed December 6, 2019).
Harp, George L., and Linden Trial. “Distribution and Status of Ophiogomphus westfalli (Odonata: Gomphidae) in Missouri and Arkansas.” Proceedings of the Arkansas Academy of Science 55 (2001): 43‒50. Online at https://scholarworks.uark.edu/jaas/vol55/iss1/7/ (accessed December 6, 2019).
Houston, Jim. “Notes on the Habitat and Distribution of the Odonata of Franklin County, Arkansas.” Proceedings of the Arkansas Academy of Science 24 (1970): 69‒73. Online at https://scholarworks.uark.edu/jaas/vol24/iss1/26/ (accessed December 6, 2019).
Huggins, Julia A., and George L. Harp. “Aquatic Macroinvertebrates of the Hiatt Prairie Region, Franklin County, Arkansas.” Proceedings of the Arkansas Academy of Science 37 (1983): 92‒94. Online at https://scholarworks.uark.edu/jaas/vol37/iss1/34/ (accessed December 6, 2019).
McAllister, Chris T., Henry W. Robison, and Michael E. Slay. “The Arkansas Endemic Fauna: An Update with Additions, Deletions, a Synthesis of New Distributional Records, and Changes in Nomenclature.” Texas Journal of Science 61 (2009): 203–218.
McGary, J. L., and George L. Harp. “The Benthic Macroinvertebrate Community of the Greer’s Ferry Reservoir Cold Tailwater, Little Red River, Arkansas.” Proceedings of the Southeastern Association of Game and Fish Commission 26 (1972): 490‒500.
Merritt, R. W., Kenneth W. Cummings, and M. B. Berg, eds. An Introduction to the Aquatic Insects of North America. 4th ed. Dubuque, IA: Kendall-Hunt Publishing Company, 2008.
Needham, J. G., and M. J. Westfall. A Manual of the Dragonflies of North America. Springfield, IL: C. C. Thomas, 1955.
Odonata Central. https://www.odonatacentral.org/ (accessed December 6, 2019).
Paulson, Dennis R., and Sidney W. Dunkle. A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution. 2018 ed. N.p.: 2018.
Rickett, John D. “An Update of Arkansas Odonata (Anisoptera).” Proceedings of the Arkansas Academy of Science 30 (1976): 73‒74. Online at https://scholarworks.uark.edu/jaas/vol30/iss1/27/ (accessed December 6, 2019).
Robison, Henry W. “Biodiversity of Seeps and Springs on the Caddo Ranger District, Ouachita National Forest, Arkansas.” Final Report to USDA Forest Service, Ouachita National Forest, 2003.
Robison, Henry W., and Robert T. Allen. Only in Arkansas: A Study of the Endemic Plants and Animals of the State. Fayetteville: University of Arkansas Press, 1995.
Robison, Henry W., and George L. Harp. “A Pre-Impoundment Limnological Study of the Strawberry River in Northeastern Arkansas.” Proceedings of the Arkansas Academy of Science 25 (1971): 70‒79. Online at https://scholarworks.uark.edu/jaas/vol25/iss1/19/ (accessed December 6, 2019).
Robison, Henry W., and Chris T. McAllister. “The Arkansas Endemic Flora and Fauna: An Update with 13 Additional Species.” Journal of the Arkansas Academy of Science 69 (2015): 78–82. Online at http://scholarworks.uark.edu/jaas/vol69/iss1/16/ (accessed December 6, 2019).
Robison, Henry, Chris McAllister, Christopher Carlton, and Robert Tucker. “The Arkansas Endemic Biota: With Additions and Deletions.” Journal of the Arkansas Academy of Science 62 (2008): 84–96. Online at http://scholarworks.uark.edu/jaas/vol62/iss1/14/ (accessed December 6, 2019).
Robison, Henry W., and Kenneth L. Smith. “The Endemic Flora and Fauna of Arkansas.” Proceedings of the Arkansas Academy of Science 36 (1982): 52–57. Online at http://scholarworks.uark.edu/jaas/vol36/iss1/17/ (accessed December 6, 2019).
Westfall, M. J., Jr. “A New Species of Gomphus from Arkansas (Odonata; Gomphidae).” Florida Entomologist 58 (1975): 91‒95.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College

 Arkansas Dragonflies
Arkansas Dragonflies  Damselflies
Damselflies 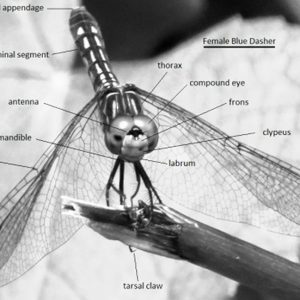 Dragonfly Morphology
Dragonfly Morphology 



Comments
No comments on this entry yet.