calsfoundation@cals.org
Dipteran Parasites
aka: Parasitic Dipterans
aka: flies
aka: mosquitos
aka: gnats
The order Diptera belongs to the Phylum Arthropoda and Class Insecta. The order ranks number two among all insect orders—only behind beetles (Coleoptera)—with about 125,000 described species (there are an estimated 1,000,000 total species), many of which are considered parasitic or serve as vectors for diseases. There are two main groups (suborders): the Nematocera with seven infraorders and Brachycera with six infraorders. Dipterans—including the sixty species of mosquitoes that occur in Arkansas and Missouri—can be irritating to humans and harmful to livestock and other animals. A major repository of voucher specimens of dipterans in Arkansas is the Entomology Arthropod Museum at the University of Arkansas in Fayetteville (Washington County). It houses the largest research and reference collection of insects and other arthropods in the state.
Primary morphological characteristics of the order include one pair of wings on the mesothorax and one pair of halteres (balance organs) on the metathorax (derived from a reduced pair of wings); however, some species have lost wings secondarily. Some taxa (families Simulidae and Tabanidae) display compound eye sexual dimorphism wherein the females have eyes separated on the top of the head (dichoptic eyes), while the males have no separation (holoptic eyes).
Representatives in the suborder Nematocera possess antennae with many segments (several are filamentous); many males have very elaborate (plumose) antennae to detect female pheromones, although females tend to have simple antennae. Nematoceran wings have many veins (primitive condition), and larvae have a well-developed head capsule. The larvae are also quite active, and many are free-swimming. The majority of species include larvae and pupae that are aquatic or develop in very moist soils. Representative parasitic families of this order include the Ceratopogonidae (biting midges), Culicidae (mosquitos), Psychodidae (sand flies and moth flies), and Simulidae (black flies).
Simulidae (a.k.a. Black Flies)
The family Simulidae includes the so-called black flies (or turkey gnats, buffalo gnats) with over 1,720 named species within about twenty-six genera. They are mostly small (one to five millimeters long), dark flies (though some species are lighter in color) that occur in temperate or subarctic environments. Many species have a humpback appearance, antennae possess eleven segments (without hairs), and the ocelli are absent. Their wings are wide with well-developed anterior veins but with poor venation elsewhere. Females have serrated mandibles used to cut skin, but some females also feed on nectar; males have reduced mouthparts and feed solely on nectar. Simulids have a large pair of compound eyes, with the females being dichoptic whereas the males are holoptic. The buffalo gnat, Cnephia pecuarum, is one of the most important livestock pest species of the black fly group in Arkansas. For example, a severe outbreak of C. pecuarum in 1931 caused the death of over 1,000 mules along the lower Mississippi River.
In the life cycle of a typical Simulium sp., females require a blood meal for proper ovarian development. Following copulation with the male in flight, they enter well-oxygenated flowing water and, with their ovipositor submerged, lay between 150 to 800 eggs per oviposition. A larva hatches in one to four days and attaches by a posterior disc-like sucker (“anal sucker” or “posterior circlet”) to a silken mat it produces and hangs downstream, where it filter feeds using fanlike projections around the mouth. There are six to nine larval instars in which each individual eventually spins a cocoon and transforms into a pupa. In two to six days to several weeks, the adult emerges.
Among worldwide arthropods, black flies are ranked third in importance as vectors of disease. Some species are important pests of livestock, wildlife, and humans, and, in large numbers, can kill their hosts. Some serve as vectors of noted human parasites, such as the filarial worm, Onchocerca volvulus, that causes river blindness (onchocercosis). There are other simulid vectors, including Simulium rugglesi of Leukocytozoon simondi infecting blood cells of waterfowl (anseriform) birds and several Simulium spp. (turkey gnats) that transmit L. smithi that infects turkeys. In Arkansas, duck or turkey malaria (leucocytozoonosis) is a disease transmitted by black flies; a major outbreak occurred in turkeys in 1970. In addition, between 2008 and 2009, losses to leucocytozoonosis in commercial turkey production occurred in the Arkansas River Valley area of the state.
Culicidae (a.k.a. Mosquitoes)
The family Culicidae comprises the mosquitos, with over 3,500 described species. Some representatives are host specific, whereas others are generalists. Mosquitoes possess slender wings with scales, an elongate proboscis where the labium encloses elongated mandibles, maxillae, hypopharynx, and the labrum/epipharynx. The proboscis is inserted into the dermal tissues of hosts and functions to imbibe blood by females; males feed on nectar and cannot suck blood. The antennae are long, with fourteen to fifteen segments, and males have plumose antennae to detect pheromones; females have simple antennae. They have long, slender legs and possess numerous appressed scales on their thorax, legs, abdomen, and wing veins; there are also fringe scales along the posterior margin of wings.
In the typical mosquito life cycle, mating occurs shortly after emergence from pupae. Females usually mate once and store sperm in their spermathecal; most require a blood meal for maturation of eggs in their ovaries. Oviposition takes place in water within boat-shaped rafts, or individually, or in small numbers in water or the soil, depending upon the species. Females may take one to two more blood meals, with egg maturation and oviposition after each feeding. Eggs hatch either soon or, as in other species, following flooding or snow melting. Larval mosquitoes are termed “wigglers” and are found in various standing water sources. Some larvae possess a siphon (breathing tube at posterior end), a well-developed head, and compound eyes. There are usually four larval instars, and most are filter feeders, although some are predators on other insects. Following pupation, the pupae (termed tumblers) periodically stick breathing tubes from their thorax (trumpets) above water and tumble to the bottom of the water source if they detect any disturbance. After two to three days, the adults emerge, and females are ready to serve as vectors or simply take a blood meal.
About sixty species of mosquitoes occur in Arkansas and Missouri. Several species are vectors of some very important diseases such as malaria (Plasmodium spp.), transmitted by Anopheles spp. In addition, some filarid parasites such as the dog heartworm (Dirofiliaria immitis) are transmitted by mosquitos (Aedes spp.) as well as arboviruses such as avian pox, yellow fever, dengue fever, various types of encephalitis, West Nile, and Zika. Zika is carried by Aedes aegypti, a mosquito found in Arkansas, and has been reported in several people who traveled to the state. Another mosquito, Aedes albopictus (the Asian tiger mosquito), is a locally abundant species that has the potential to restrict outdoor human activities within cities located in the Ozark Mountains Physiographic Region of Arkansas, an area that historically lacked any such problem with pestiferous mosquitoes. It serves as a vector of several viral diseases of humans, including dengue fever and encephalitis. The northern (Culex pipiens) and southern (C. quinquefasciatus) house mosquitoes commonly bite humans as they sleep at night. An economically important species in Arkansas, the dark ricefield mosquito (Psorophora columbiae) can, in large numbers, cause weight loss and a reduction in milk production in livestock resulting from its blood-feeding activity; it has also been reported to kill livestock. Psorophora columbiae can also cause extreme annoyance to humans. This mosquito is an important vector of Venezuelan equine encephalitis and anaplasmosis in cattle.
Several cities in the state have budgets for mosquito control. These measures are especially important in helping control mosquitoes in areas with standing water and in cities of eastern Arkansas near rice fields. The Arkansas Department of Health suggests people who want to avoid mosquito bites use an insect repellent containing DEET, picaridin, IR3535 (ethyl butylacetylaminopropionate), or oil of lemon eucalyptus. In addition, wearing long-sleeved shirts and pants, using window and door screens to keep mosquitoes outside, and emptying standing water from any type of containers are helpful measures. The massive flooding caused by Hurricane Irma in September 2017 in southeastern Texas prompted the state to spray over two million acres in that region to help control mosquitoes.
Phlebotominae (a.k.a. Sand Flies)
The family Psychodidae includes the subfamily Phlebotominae or sand flies. Although several are important as vectors of diseases and parasites (like Leishmania), none from the genus Lutzomyia are known from Arkansas. However, at least five species have been reported from adjacent Texas, including Lutzomyia anthophora, L. californica, L. cubensis, L. diabolica, and L. texana.
Ceratopogonidae (a.k.a. Biting Midges)
The family Ceratopogonidae includes the biting midges, also locally referred to as “no-see-ums” or “punkies.” There are about 4,000 or more described species within 125 genera. These are very small dipterans, less than one mm in length, but they are aggressive. These midges are primarily day feeders in the summer months, and most feed on insects. However, some feed on cold-blooded (poikilothermic) vertebrates, and four genera (Austroconops, Culicoides, Forcipomyia, Leptoconops) are known to feed on mammals, including humans. The genus Culicoides has about 1,200 species and includes the most important members that cause biting problems. Some serve as vectors of diseases, including bluetongue (an orbivirus) and an epizootic hemorrhagic disease in wild ruminants such as deer. They can also serve as vectors for some parasites, such as filarids (e.g., several Mansonella spp., Onchocerca spp.), malarias, Leukocytozoon spp., Haemoproteus spp., and some Hepatocystis spp. Biting midges can be a nuisance to people who spend time outdoors during early morning and evenings, and even during the daytime on cloudy days when winds are calm. High elevations do not seem to limit their distribution, as campers have complained of bites when they were frequenting sites atop Rich Mountain at 817 meters (2,680 feet) in Polk County. They will readily bite humans; the bites are irritating, painful, and can cause long-lasting painful misery for some people. Other mammals such as horses can experience equine allergic dermatitis, a localized allergic reaction to biting midges that affects the withers, mane, tail, or ears of sensitive individuals.
Brachycera
The suborder Brachycera includes about 120 families of flies that tend to be robust. Their antennae are reduced to three segments with a pointed terminal segment, and wing venation is reduced. Several species are brilliantly colored brown, black, green, or orange. The larval cephalic region is usually reduced, vestigial, or retractable, and these larvae are active and normally predaceous. Some representative families include the Tabanidae (horse flies and deer flies), Chloropidae (eye gnats), Muscidae (house flies and stable flies), Glossinidae (tsetse flies), Calliphoridae (blow flies), Hippoboscidae (louse flies), Sarcophagidae (flesh flies), and Oestridae (bot flies).
An important parasite transmitted by deer flies (Chrysops spp.) is the eye worm, Loa loa. In addition, Tabanus spp. (horse flies) and tsetse flies (Glossina spp.) can transmit several species of Trypanosoma, including T. evansi that causes surra in animals and various other trypanosomes that cause sleeping sickness in animals and humans. None of these diseases are found in these vectors in the United States, although horseflies can be a biting nuisance to humans during outdoor activities in Arkansas and elsewhere.
Examples of the large family Muscidae include the common house fly (Musca domestica), a statewide inhabitant of various locales in Arkansas and a nuisance in human dwellings and other buildings. These flies can be very plentiful, as females lay five to six batches of about 100 eggs/batch in a lifetime. Larval maggots have three instars, and, depending upon food availability and temperature, development is complete in three to twenty-four days. The stable fly, Stomoxys calcitrans, also widespread in Arkansas, looks much like a house fly, but it bites and so is often called the “biting house fly.” Both sexes feed on blood during the day and bite a variety of livestock, domestic animals, and humans. This painful biter possesses piercing mouthparts that protrude from under its head to allow taking blood meals. They breed in decaying vegetation (compost piles) and sometimes manure. Stable flies can transmit diseases phoretically, which include anthrax, brucellosis, fowl pox, and others. The horn fly, Haematobia irritans, is mostly a blood-feeding irritant of cattle but can be a significant nuisance of equines pastured with or near cattle. The face fly, Musca autumnalis, is a non-biting fly that resembles the house fly. However, it can still be a severe annoyance, as adult females persistently feed on secretions around animals’ eyes, nose, or wounds.
Blow flies include brightly colored metallic blue, green, or tan species, with adults producing a low buzzing sound. They possess larvae that burrow into the skin and destroy dermal tissue/hides, especially of cattle. The primary screw worm, Cochliomyia hominivorax, occurs in the Western Hemisphere, although it has been eradicated in most areas north of Mexico by release of sterile males (as females only mate once). Larval calliphorid flies have been reported from a dermal wound (case of myiasis) of a three-toed box turtle (Terrapene carolina triunguis) from Saline County, Arkansas. There are several types of myiasis: facultative myiasis in which the normally free-living maggot can successfully establish parasitism by gaining access to the host accidentally; obligatory myiasis, which is necessary for completion of the life cycle; and pseudomyiasis, in which eggs/larvae are ingested and larvae may reside for a time enterically. Many species of blow flies are cutaneous inhabitants, either ingesting necrotic or live tissues, whereas others may be endoparasitic in gastric, urogenital, nasopharyngeal, or ophthalmic tissues.
Louse flies look much like ticks; however, they possess only six legs. Wings have been lost by the females of most species, whereas males have usually retained wings; in some species, though, both sexes possess wings. Both males and females feed on blood. The sheep ked, Melophagus ovinus, is a cosmopolitan species that glues its puparium to wool (females produce about a dozen offspring in their lives) where it spends its entire life on a host. It has a painful bite, and heavy infestations may result in emaciation, scarring, and anemia. There is no doubt that this occurs on many domesticated animals in Arkansas.
Flesh flies produce live larvae in wounds or on carcasses; many species infest invertebrates. One species, Sarcophaga cistudinis, occurs on turtles and is often found embedded in the dermis around the neck. In Arkansas, egg clutches of the prairie lizard, Sceloporus consobrinus, have been reported to be infested in Randolph County with larvae of the flesh fly, Eumacronychia nigricornis. Most larval specimens were found outside damaged lizard eggs (in the soil beneath the eggs), where they were feeding on yolk that oozed out of the perforations.
The bot flies are included within the subfamily Cuterebrinae (rodent or skin bots), which are large dark flies that lay eggs near orifices. Larvae enter an orifice, tunnel under the skin, cut out an air hole, and ingest live tissues. There are many different species in mammals, including Cuterebra spp. that are found in deer, rodents, and rabbits; they can also infest birds. In Arkansas, a larval Cuterebra sp., was reported from the neck of a cottontail rabbit, Sylvilagus floridanus, from Hot Spring County.
Stomach bots (subfamily Gasterophilinae) include females that lay eggs in hair of horses, elephants, and rhinoceroses; three species have been introduced into the United States with horses. In Arkansas, the horse bot (Gastrophilus intestinalis) has eggs that enter horses’ mouths after being licked off; larvae hatch quickly, penetrate the tongue epithelium, and tunnel to the stomach through epithelium. They emerge and attach with hooks and after two molts, detach in spring and early summer, are passed out in feces, and pupate in the soil. Adults resemble bees in that they are about the same size, hairy-bodied, and short-lived; they possess non-functional mouthparts and do not feed. Adult activity begins in warm weather and ends at the first frost.
The nasal bots, pharyngeal bots, and head maggots (subfamily Oestrinae) are large adult flies that do not feed. For example, the eggs of the sheep nasal bot (Oestrus ovis) are laid in late summer or fall near the nostrils of sheep and goats worldwide. Larvae crawl up into sinuses, where they attach and feed on the sinus mucosa. Eventually, larvae migrate back down, drop to the ground, and pupate in spring. In infested hosts, sneezing, head shaking, and nasal discharge can occur.
Bat flies are a group of highly specialized dipterans that are obligate ectoparasites on bats. There are two families: the Nycteribiidae (bat spider flies) and Streblidae (bat flies). Members of both families show reduced compound eyes, no ocelli, a spider-like orientation of their legs, and adenotrophic vivipary. Strebilids have been reported on endangered gray bats (Myotis grisescens) and Ozark big-eared bats (Corynorhinus townsendi ingens) in Blue Heaven Cave of Marion County and on C. t. ingens in undisclosed northwestern Arkansas caves.
For additional information:
Arnett, R. H., Jr. American Insects. 2nd ed. Boca Raton, FL: CRC Press, Boca Raton, 2000.
Borror, Donald J., and R. E. White. A Field Guide to the Insects of America North of Mexico. Boston: Houghton Mifflin Company, 1970.
Bowles, D. E., and D. D. Pinkovsky. “Occurrence of Larval Black Flies and Horse Flies in an Ozark Headwater Stream.” Southwestern Naturalist 38 (1993): 86–87.
Carlton, Chris E., and Jack L. Lancaster Jr. Horse and Deer Flies of Arkansas (Insecta, Diptera, Tabanidae). University of Arkansas (Fayetteville) Agricultural Experiment Station Bulletin 948, 1995.
Crockett, R. T. “Arbovirus Surveillance and Control of Arkansas Mosquitoes.” MS thesis, University of Arkansas, 2002.
Darsie, R. F., and R. A. Ward. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville: University Press of Florida, 2005.
Dunivan, J. D., C. Renn Tumlison, and V. Rick McDaniel. “Cave Fauna of Arkansas: Further Records.” Proceedings of the Arkansas Academy of Science 36 (1982): 87–88. Online at http://scholarworks.uark.edu/jaas/vol36/iss1/28/ (accessed September 22, 2021).
Johnson, Norman F., and C. A. Triplehorn. Borror and DeLong’s An Introduction to the Study of Insects. 7th ed. Belmont, CA: Thomson Brooks/Cole, 2005.
Dick, Carl W. Streblidae. In Encyclopedia of Parasitology, 4th ed. Edited by H. Mehlhorn. Berlin: Springer-Verlag Publishing, 2014.
Dick, Carl W., and R. Pospischil. Nycteribiidae. In Encyclopedia of Parasitology, 4th ed. Edited by H. Mehlhorn. Berlin: Springer-Verlag Publishing, 2014.
Dittmar, Katharina, Megan L. Porter, Susan Murray, and Michael F. Whiting. “Molecular Phylogenetic Analysis of Nycteribiid and Streblid Bat Flies (Diptera: Brachycera, Calyptratae): Implications for Host Associations and Phylogeographic Origins.” Molecular Phylogenetics and Evolution 38 (2006): 155–170.
Entomology Arthropod Museum, University of Arkansas. https://arthropod.uark.edu/ (accessed September 22, 2021).
Hall, M., and R. Wall. “Myiasis of Humans and Domestic Animals.” Advances in Parasitology 35 (1995): 257–334.
Hilburn, L. R., and Lynita M. Cooksey. “Patterns of Genetic Variability in Anopheles quadrimaculatis (sensu stricto) (Diptera: Culicidae) Populations in Eastern Arkansas.” Journal of Medical Entomology 41 (2004): 40–46.
Horsfall, W. R. “Mosquitoes of Southeastern Arkansas.” University of Arkansas (Fayetteville) Agricultural Experiment Station Bulletin 351 (1938): 45-46.
Jamieson, David H., and Larry A. Olson. “Recent Establishment of the Asian Tiger Mosquito (Aedes albopictus) in Independence County, Arkansas.” Proceedings of the Arkansas Academy of Science 49 (1995): 80–81. Online at http://scholarworks.uark.edu/jaas/vol49/iss1/19/ (accessed September 22, 2021).
Jamieson, David H., Larry A. Olson, and J. D. Wilhide. “A Larval Mosquito Survey in Northeastern Arkansas Including a New Record for Aedes albopictus.” Journal of the American Mosquito Control Association 10 (1994): 236–239.
Jones, J. W., and M. V. Meisch. “Potential Mosquito Vectors of Dog Heartworm, Dirofilaria immitis, in Eastern Arkansas.” Southwestern Entomologist 18 (1993): 19–23.
Krafsur, E. S., C. J. Whitten, and J. E. Novy. “Screwworm Eradication in North and Central America.” Parasitology Today 3 (1987): 131–137.
Lancaster, Jack L., Jr., and C. D. McNeal Jr. “A Preliminary Encephalide Virus Survey in Arkansas.” Journal of the Kansas Entomological Society 44 (1971): 181–185.
Loftin, Kelly M., and Ricky F. Corder. Arthropod Pests of Equines. Fayetteville: University of Arkansas Division of Agriculture Publication MP484-PD-6-10N, 2015.
McAllister, Chris T. “Phaenicia (Diptera: Calliphoridae) Myiasis in a Three-Toed Box Turtle, Terrapene carolina triunguis (Reptilia: Emydidae), from Arkansas.” Texas Journal of Science 39 (1987): 377–378.
Meisch, Max V. “The Dark Ricefield Mosquito Psorophora columbiae.” Wing Beats 5 (1994): 8.
Meisch, Max V., A. L. Anderson, Robert L. Watson, and Larry A. Olson. “Mosquito Species Inhabiting Rice Fields in Five Rice Growing Regions of Arkansas.” Mosquito News 42 (1980): 341–346.
Mullen, G. R., and Lance A. Durden. Medical and Veterinary Entomology. San Diego, CA: Academic Press/Elsevier Science, 2002.
Revels, M. R. “Protocalliphora (Diptera: Calliphoridae) Parasitism of Breeding Birds in Arkansas: Ecological Relationships and Effects on Hooded Warbler (Wilsonia cirtrina) Breeding Biology.” PhD diss., University of Arkansas, 1997.
Roberts, Larry S., and John Janovy Jr. Foundations of Parasitology. 9th ed. Boston: McGraw-Hill Higher Education, 2012.
Savage, H. M., G. C. Smith, C. J. Mitchell, R. G. McLean, and Max V. Meisch. “Vector Competence of Aedes albopictus from Pine Bluff, Arkansas, for a St. Louis Encephalitis Virus Strain Isolated During the 1991 Epidemic.” Journal of the American Mosquito Control Association 10 (1994): 501–506.
Sealander, John A., and Howard Young. “Preliminary Observations on the Cave Bats of Arkansas.” Journal of the Arkansas Academy of Science 7 (1955): 21–31. Online at http://scholarworks.uark.edu/jaas/vol7/iss1/10/ (accessed September 22, 2021).
Schwardt, H. H. Horseflies of Arkansas. Fayetteville: University of Arkansas Agricultural Experiment Station Bulletin 332, 1936.
Trauth, Stanley E., and G. R. Mullen. “Additional Observations on Sarcophagid Fly Infestations of Sceloporus undulatus (Sauria: Iguanidae) Egg Clutches in Arkansas.” Southwestern Naturalist 35 (1990): 97–98.
Trout, Rebecca T., C. D. Steelman, and A. L. Szalanski. “Phylogenetics and Population Genetics of the Louse Fly, Lipoptena mazamae, from Arkansas, USA.” Medical and Veterinary Entomology 24 (2010): 258–265.
Tumlison, Renn, Chris T. McAllister, Henry W. Robison, Matthew B. Connior, D. Blake Sasse, Donald G. Cloutman, Lance A. Durden, Charles R. Bursey, Thomas J. Fayton, S. Schratz, and M. Buckley. “Vertebrate Natural History Notes from Arkansas, 2017.” Journal of the Arkansas Academy of Science 71 (2017).
Weathersbee, A. A., III, and Max V. Meisch. “Dispersal of Anopheles quadrimaculatus (Diptera: Culicidae) in Arkansas Ricelands.” Environmental Entomology 19 (1990): 961–965.
Wilson, J., and David H. Jamieson. “Presence of the Asian Tiger Mosquito (Aedes albopictus) in Northwest Arkansas.” Journal of the Arkansas Academy of Science 64 (2010): 151–152. Online at http://scholarworks.uark.edu/jaas/vol64/iss1/31/ (accessed September 22, 2021).
Chris T. McAllister
Eastern Oklahoma State College
 Dipterans
Dipterans Science and Technology
Science and Technology Botflies
Botflies 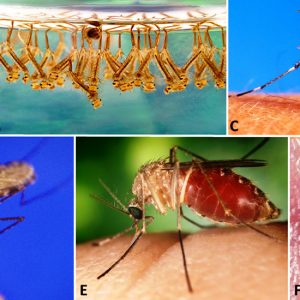 Dipteran Parasites
Dipteran Parasites 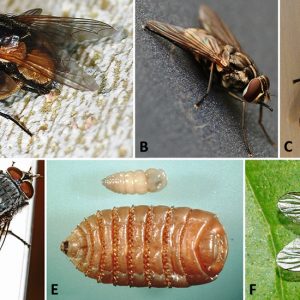 Dipteran Parasites
Dipteran Parasites 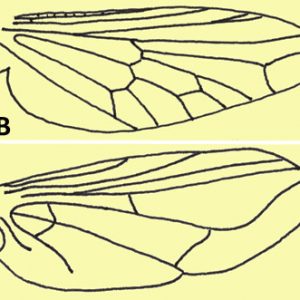 Dipteran Wings
Dipteran Wings  Flies
Flies 



Comments
No comments on this entry yet.