calsfoundation@cals.org
Cnidarians
aka: Hydroids
aka: Corals
aka: Jellyfishes
aka: Sea Anemones
Cnidarians (hydroids, jellyfishes, corals, and sea anemones) form a diverse phylum (Cnidaria, old Phylum Coelenterata) that contains more than 10,000 species. The phylum also includes the parasitic Myxozoa. Typical cnidarians inhabit aquatic (predominantly marine) environments. Cnidarians are divided into two major groups: the Anthozoa (corals, sea anemones, and sea pens), which live as sessile polyps, and the subphylum Medusozoa (Hydra, jellyfishes, and sea wasps), many of which form a free-swimming medusa as well as polyps. There are five main classes: Anthozoa, Cubozoa, Hydrozoa, Scyphozoa, and Staurozoa. Only a few cnidarians can be found in Arkansas, including a jellyfish seen in lakes and rivers.
In terms of evolutionary relationships, modern molecular phylogenetic results support the notion that anthozoans represent the first major branch of the Cnidaria. However, because of difficulties owing to imperfect preservation of fossil cnidarian candidates and their putative taxonomic assignments, little is known about forms ancestral to those of living groups. Morphological characteristics of a stem-group cnidarian, Cambroctoconus orientalis, from the mid-Cambrian of China, suggest that the colonial occurrence with polyps of octoradial symmetry is the plesiomorphic condition of the Cnidaria and appeared earlier than the jelly-like mesenchyme during the course of evolution.
In the fossil record, exceptionally preserved cnidarian jellyfish fossils from the Middle Cambrian (about 505 million years old) have been reported from the Marjum Formation of Utah. Also, a variety of putative embryonic, larval, and adult microfossils of most probable cnidarian affinity are from the Precambrian phosphorite deposits of southwest China, which may predate the Cambrian radiation by 25 to 45 million years. There are a few cubozoan fossils known from the well-known Carboniferous Mazon Creek Formation of Illinois. Fossils of possible soft-bodied polyps are rare, and modern corals are dated back only to the Middle Triassic.
In general, cnidarians are characterized as carnivorous organisms, most feeding by means of external radial symmetry (except internal asymmetries and bilaterality are displayed in some groups) and tentacles with specialized stinging cells called nematocytes with cnidocytes located in the epidermis to capture prey. Nematocysts serve as “mini-harpoons,” and the cnidocil (a hair-like sensory process projecting from the surface of a cnidoblast) senses movement and acts like a “trigger” that can inject poison, coil around prey, or become adhesive. Cnidarians also have a sac body plan with a gastrovascular cavity, and the water within the gastrovascular cavity acts as a hydrostatic skeleton. There are no true organs, and adult structures form in the diploblastic (ectoderm and endoderm) tissues. The life cycle includes two body forms: a sessile polyp and a floating medusa. Their food and waste go in and out a single opening, so it is an incomplete system that acts as both a mouth and an anus. Gas exchange and excretory systems are absent. The nervous system is a nerve “net” without a central nervous system or brain, but there are sense organs, including statocysts (equilibrium organs) and ocelli (photosensitive organs).
Cnidarians are the only animals among the four basal metazoan phyla (Porifera, Placozoa, Cnidaria, and Ctenophora) that have evolved advanced eyes. Many hydrozoan medusa have simple eyes at the rim of the bell, whereas the most sophisticated eyes are found in the cubozoan jellyfish. Box jellyfish have a total of twenty-four eyes arranged in a set of four sensory structures (rhopalia), each consisting of two lens eyes and four bilaterally paired pigment cup eyes. Cnidarians have multiple ciliary opsins, the light-sensitive receptors or proteins found in photoreceptor cells.
Reproduction in cnidarians is either: (1) asexual by budding or longitudinal fission and (2) sexual (usually dioecious) with separate sexes. However, cnidarians can also have monoecious sexual reproduction with both male and female gonads in a single individual, resulting in ciliated planulae (larvae).
The largest class of cnidarians is the Anthozoa (the flower animals); the class is all marine and comprises about 6,000 species. This group includes the soft and stony colonial corals, solitary sea anemones, gorgonians (sea fans, pens, and whips), and sea pansies. Adult anthozoans are almost always attached to the seabed, while their larvae are dispersed as part of the plankton. Sea anemones possess sessile polyps that live attached to the substrate and lack a medusa stage. Corals are colonial polyps composed of a house made of calcium carbonate; they can form extensive reefs, such as the Great Barrier Reef of Australia. Anthozoans are carnivores that capture prey items with their tentacles. Unlike other cnidarians, anthozoans do not have a medusa stage in their development. Instead, they release their gametes (sperm and eggs) directly into the water. Some anthozoans can also reproduce asexually through budding or by breaking in pieces.
The Cubozoa is a small class (about fifty species) of cube jellyfishes characterized by their square shape, thick mesoglea, four evenly spaced stalks from which tentacles hang, and well-developed eyes. The medusa stage is the conspicuous form, and these jellyfishes are strong swimmers and voracious predators that primarily feed on fishes. Stings from some produce extremely toxic venom (more potent than cobra venom) known to be fatal to humans, including those from the sea wasp (Chironex fleckeri) of northern Australian and New Guinea waters ranging farther northward to the Philippines and Vietnam. This cubozoan has been described as “the most lethal jellyfish in the world”—in Australia, twice as many people die annually from box jellies than from sharks. A small sting from another cubozoan, the Irukandji jellyfish (Carukia barnesi), causes Irukandji syndrome in humans, which can be potentially fatal. Every summer in Australia, about sixty people are hospitalized with this syndrome. Quick medical intervention and intravenous administration of pethidine (meperidine) hydrochloride is used to treat extreme pain in patients with this syndrome.
The most variable class is the Hydrozoa. It is mostly a colonial marine group (a few genera live in freshwater) with an asexual polyp and sexual medusa stage, although a number of species have only one or the other. It includes about 3,800 species, and some well-known examples include freshwater hydras (Hydra spp.), fire corals (Millepora), Obelia, the Portuguese man-o-war (Physalia physalis), and freshwater medusa, Craspedacusta sowerbii (which occurs in Arkansas). Fire corals are capable of inflicting predominantly local pain, usually described as stinging or burning, and possible rashes to humans. The venomous Portuguese man-o-war, with its long tentacles, delivers a painful sting, which is powerful enough to kill fish or, rarely, humans.
The true jellyfishes make up the exclusively marine class of about 200 species of Scyphozoa. In diameter, they typically range from 2 to 40 cm (0.8 to 15.8 in.), but the largest species, Cyanea capillata, can reach 2 m (6.6 ft.) across. A good example of this group is the moon jellyfish (Aurelia aurita). These jellyfishes serve as food for other organisms. Other examples include the genera Cassiopeia, Cyanea, and Rhizostoma. The former is a small tropical jellyfish strange among jellyfishes. It lies on the bottom in shallow waters with its very reduced mouth with the tentacles oriented upward. The jellyfish gets most of the nutrition it requires from symbiotic dinoflagellates that live inside its body tissues. Cyanea capillata, the giant jellyfish or lion’s mane jellyfish, can be quite large. In fact, the largest recorded specimen, found washed up in 1870 on the shore of Massachusetts Bay, had a bell with a diameter of 2.3 m (7 ft., 6 in.) and tentacles 37 m (120 ft.) long. Rhizostoma pulmo, commonly known as the barrel jellyfish, is found in the northeast Atlantic, the Adriatic, the Mediterranean Sea, Black Sea, Sea of Azov, and the southern Atlantic off the western South African coast. It is typically up to 60 cm (24 in.) in diameter, but exceptional specimens can reach 90 cm (35 in.).
Most species of scyphozoans have two life history phases, including the planktonic medusa or jellyfish form, which is most evident in the warm summer months, and an inconspicuous, but longer-lived, bottom-dwelling polyp form, which seasonally gives rise to new medusae. Most of the large, often colorful, and conspicuous jellyfish found in coastal waters throughout the world are Scyphozoa. Scyphomedusae are found throughout the world’s oceans, from the surface to great depths. As medusae, they eat a variety of crustaceans and fish, which they capture using stinging cells. However, some species are instead filter feeders, using their tentacles to strain plankton from the water.
The odd jellyfishes belong to the benthic class Staurozoa, small sessile individuals with a stalked polyp stage. It contains one extant order, the Stauromedusae (stalked primitive jellyfishes) and includes about fifty species. These small cnidarians (1–4 cm [0.4–1.6 in.]) live in marine environments, usually attached to gravel, rocks, or seaweeds. They have a large anti-tropical distribution, a majority found in near-shore, boreal, or polar cold shallow waters. Few staurozoans are found in warmer tropical and subtropical waters of the Atlantic, Indian, and Pacific Ocean basins, but the majority are known from the Northern Hemisphere. Anatomically, they have a trumpet-shaped body, oriented upside-down in comparison with other jellyfish, with the tentacles projecting upward, and have the stalk located in the center of the umbrella. They are unique among jellyfish medusa, as they develop directly from benthic planula larvae and do not have an alternation of polyp and medusa life cycle phases but are instead interpreted as an attached medusa stage—a lifestyle more similar to that of polypoid forms. They are also capable of changing locations but normally attach with the stalk and adhesive pads to solid objects like kelp and rocks.
One common freshwater jellyfish, Craspedacusta sowerbii, is a translucent whitish or greenish-tinged hydrozoid form that measures about 5 to 25 mm (about 1 in.) in diameter. It was first recorded and described from the “Victoria regia” (Victoria amazonica) tank at the Royal Botanic Society’s Gardens in Regent’s Park, London. This invasive freshwater jellyfish now lives in waters on every continent except Antarctica, and perhaps no other aquatic species on Earth has spread so far and wide. It is now common in temperate climates almost globally, occurring frequently in Australia, Eurasia, and North and South America, occupying a range of freshwater habitats. However, the native range is the Yangtze River in China, where it typically inhabits shallow pools. This jellyfish was likely transported in polyp form with ornamental aquatic plants, especially water hyacinth (Eichhornia crassipes), from its native habitat to the Philadelphia, Pennsylvania, area. Since it was first found in the United States in 1880, C. sowerbii has spread to most provinces in Canada and forty-five states (including Hawaii) and the District of Columbia, excepting the colder states of Alaska, Montana, North Dakota, South Dakota, and Wyoming. In the United States, polyps and resting bodies were probably translocated accidentally with stocked fish and aquatic plants or by waterfowl. Where C. sowerbii has been introduced, it is most commonly found in shallow, slow-moving, or stagnant artificial water bodies such as ornamental ponds, calm reservoirs, gravel pits, and quarries. In North America, it has also been reported in large river systems, including the Allegheny, Ohio, and Tennessee river systems, as well as natural lakes, aquaria, and ornamental ponds. It lives predominantly as a polyp most of the year in small lakes, ponds, and old water-filled quarries, and the medusa stage occurs during July through October. In Arkansas, this jellyfish has been documented in locations across the state, including the following lakes and reservoirs: Beaver, Bull Shoals, Dardanelle, DeGray, Dierks, Greers Ferry, Greeson, Maumelle, Ouachita, Norfork, and Wedington. Other bodies of water include rivers such as the Buffalo, Cache, Illinois, Little Missouri, Little Red, lower Arkansas, lower Black, lower Red, lower St. Francis, Mulberry, Ouachita, upper Saline, and White.
Craspedacusta sowerbii is easily identified when it takes the form of the hydromedusa, a small, bell-shaped jellyfish. It possesses five opaque-white canals (four are radial and one is medially dorsoventral), which form the gastrovascular cavity. Tentacles of varying lengths are arranged with three to seven short tentacles between longer ones that protrude from the upper margin of the velum (muscular, shelf-like structure). The total number of tentacles varies from fifty to 500. Conspicuous swarms of hydromedusae appear sporadically but are only one part of the animal’s life cycle. Craspedacusta sowerbii more often exist as microscopic dormant podocysts (“resting bodies”), frustules (larvae produced asexually by budding), planulae (larvae produced sexually by the hydromedusae), or sessile polyps, which attach to stable surfaces and can form colonies consisting of two to four individuals and measuring 5 to 8 mm (0.2 to 0.3 in.).
Craspedacusta sowerbii reproduce both sexually and asexually. They reproduce sexually as mature hydromedusae by broadcasting their gametes into the water. Fertilized eggs grow into ciliated planulae (larvae), which then settle and metamorphose into a tiny polyp stage. All-male or all-female populations of C. sowerbii are common, making sexual reproduction quite rare. They are also capable of asexual (budding) as polyps to produce hydromedusae (which are only produced sporadically), as well as either daughter polyps that remain attached to the parent, forming a colony, or frustule larvae that move to new locations before metamorphosing into new polyps. Overwintering polyps contract into resting bodies (podocysts), which are basically dormant cellular “balls” surrounded by a protective chitin-like membrane that allows them to withstand more extreme conditions than the active forms. It is believed that podocysts are transported by aquatic plants or animals to other bodies of water.
Like other cnidarians, C. sowerbii is an opportunistic predator, feeding on small organisms that come into contact with its stinging tentacles. Both polyp and hydromedusa stages use nematocysts to capture prey. There appears to be a preference for predatory zooplankton, such as the rotifer Asplanchna, larger zooplankton, and energetic prey such as Daphnia and copepods. This jellyfish may also consume fish eggs, but they are not generally considered an important predator of eggs or small fishes. Drifting with its tentacles extended, the jellyfish waits for suitable prey to touch a tentacle. Once contact has been made, nematocysts on the tentacle fire into the prey, injecting poison that paralyzes the animal, and the tentacle itself coils around the prey. The tentacles then bring the prey into the mouth, where it is released and then digested. Crayfish are considered the only important predator of the hydromedusa phase. This freshwater jellyfish is not considered dangerous to humans. Although its stings can paralyze macroinvertebrates and small fish, its small nematocysts are incapable of penetrating human skin.
Another cnidarian in Arkansas is the carnivorous green hydra, Hydra viridissima. It is a freshwater hydroid polyp that never forms a medusa and is found widely dispersed in the northern temperate zone. It is a common organism found in calm waters attached to stems of aquatic plants and undersides of leaves (including duckweed) from early spring to late autumn. The characteristic green color of this organism arises from Chlorella-like unicellular algae each occupying a vacuole in the endodermal cells. These zoochlorellae carry out photosynthesis and produce sugars that are used by H. viridissima. As a result, H. viridissima are generally less predacious than aposymbiotic hydra species. It reproduces primarily asexually by budding of lateral polyps, but it also has a sexual cycle. Depending on the taxon, polyps are either hermaphroditic or dioecious; there is no larval stage between the embryo and polyp. The embryo completes development within a cuticle from which a fully formed polyp hatches after a dormancy phase of weeks to months.
The most bizarre of the freshwater cnidarians is the enigmatic species Polypodium hydriforme. This endocellular parasite spends almost all of its pre-adult life inside its host cells as a parasite of the oocytes of acipenseriform fishes (sturgeons and paddlefish). Its hosts include the kaluga (Huso dauricus), beluga (H. huso), sterlet (Acipenser ruthenus), Russian sturgeon (A. gueldenstaedtii), fringebarbel sturgeon (A. nudiventris), starry sturgeon (A. stellatus), lake sturgeon (A. fluvescens), shovelnose sturgeon (Scaphirhynchus platorynchus), and American paddlefish (Polyodon spathula). The last three fishes are found in Arkansas, but there are no reports as of 2019 of this parasite infecting them in the state. This cnidarian potentially occurs in Arkansas because P. hydriforme has been reported from nearby Missouri in P. spathula. Polypodium traditionally has been considered a cnidarian (species of Hydrozoa) because it possesses nematocysts; however, its relationships are currently unresolved.
For additional information:
Acker, T. S., and A. M. Muscat. “The Ecology of Craspedacusta sowerbyi Lankester, a Freshwater Hydrozoan.” American Midland Naturalist 95 (1976): 323‒336.
Brussock, Peter P., Lawrence D. Willis, and Arthur V. Brown. “Flow Reduction May Explain Sporadic Occurrence of Craspedacusta sowerbyi (Trachylina) Medusae.” Proceedings of the Arkansas Academy of Science 39 (1985): 120. Online at https://scholarworks.uark.edu/jaas/vol39/iss1/30/ (accessed September 30, 2019).
Cartwright, P., S. L. Halgedahl, J. R. Hendricks, R. D. Jarrard, C. A. Marques, A. G. Collins, and B. S. Lieberman. “Exceptionally Preserved Jellyfishes from the Middle Cambrian.” PLoS One 2 (2007): e1121.
Dendy, J. S. “Polyps of Craspedacusta sowerbyi as Predators on Young Striped Bass.” Progressive Fish-Culturist 40 (1978): 5‒6.
DeVries, D. R. “The Freshwater Jellyfish Craspedacusta sowerbyi: A Summary of its Life History, Ecology and Distribution.” Journal of Freshwater Ecology 7 (1992): 7‒16.
Dodson, S. I., and S. D. Cooper. “Trophic Relationships of the Freshwater Jellyfish Craspedacusta sowerbyi Lankester 1880.” Limnology and Oceanography 28 (1983): 345‒351.
Dumont, H. J. “The Distribution and Ecology of the Fresh- and Brackish-Water Medusae of the World.” Hydrobiologia 272 (1994): 1‒12.
Edmondson, C. H. “Fresh-Water Jellyfish in Hawaii.” Science 91 (1940): 313‒314.
Hoffman, Glenn L. Parasites of North American Freshwater Fishes. 2nd ed. Berkeley: University of California Press, 2009.
Jankowski, T. “Predation of Freshwater Jellyfish on Bosmina: The Consequences for Population Dynamics, Body Size, and Morphology.” Hydrobiologia 530/531 (2004): 521‒528.
McAllister, Chris T., Donald G. Cloutman, Eric M. Leis, Alvin C. Camus, and Henry W. Robison. “A New Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Gill Arch of the Western Creek Chubsucker, Erimyzon claviformis (Cypriniformes: Catostomidae), in Arkansas, USA.” Acta Parasitologica 70 (2025). https://doi.org/10.1007/s11686-025-01001-6 (accessed March 13, 2025).
McCullough, J. D., M. F. Taylor, and J. L. Jones. “The Occurrence of the Freshwater Medusa Craspedacusta sowerbyi in Nacogdoches Reservoir, Texas and Associated Physical-Chemical Conditions.” Texas Journal of Science 33 (1981): 17‒23.
Pennak, Robert W. Fresh-Water Invertebrates of the United States: Protozoa to Mollusca. 3rd ed. New York: John Wiley & Sons, 1989.
Spadinger, R., and G. Maier. “Prey Selection and Diel Feeding of the Freshwater Jellyfish, Craspedacusta sowerbyi.” Freshwater Biology 41 (1999): 567‒573.
Suppes, V. C., and F. P. Meyer. “Polypodium sp. (Coelenterata) infection of Paddlefish (Polyodon spathula) eggs.” Journal of Parasitology 61 (1975): 772‒774.
Thorp, J. H., and A. P. Covich, eds. Ecology and Classification of North American Freshwater Invertebrates. San Diego: Academic Press, 1991.
Chris T. McAllister
Eastern Oklahoma State College
Henry W. Robison
Sherwood, Arkansas
 Science and Technology
Science and Technology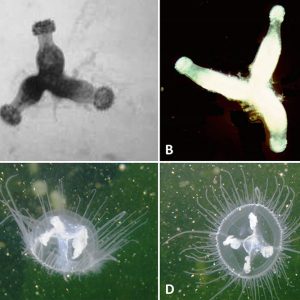 Freshwater Cnidarians
Freshwater Cnidarians  Hydra
Hydra 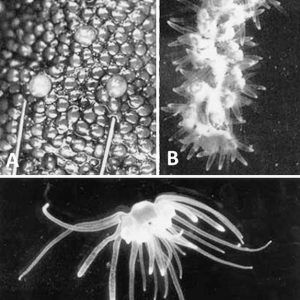 Polypodium hydriforme
Polypodium hydriforme 



Comments
No comments on this entry yet.