calsfoundation@cals.org
Ostracods
aka: Seed Shrimps
aka: Mussel Shrimps
Ostracods belong to the Class Ostracoda within the Subphylum Crustacea and Phylum Arthropoda. Commonly called “seed or mussel shrimps,” the class encompasses over 33,000 described species and subspecies, and many more remain unknown to science and await formal description. There are two subclasses with living representatives: the Myodocopa and Podocopa. The former is exclusive to marine environments but occupies the benthos as well as the plankton, whereas podocopans occur in marine, brackish, and freshwater environments and occupy almost exclusively the benthos (but also the benthopelagic zone). The main orders are the Archaeocopida, Leperditicopida, Palaeocopida, Podocopida, and Myodocopida. As of 2019, there is no comprehensive list of extant ostracods in Arkansas.
Ostracods are important fossil organisms, as they are the most common representative fossils in the fossil record. Anselme G. Desmarest (1784‒1838) of France described the first fossil ostracod, Cypris fuba. Ostracods date back to the early Ordovician Period (485.4 million years ago). Freshwater ostracods have been discovered in Baltic amber of Eocene age (44 million years ago). Due to their wide occurrence, ostracods are quite important in assessing biozonation of marine strata as well as being an indicator of paleoenvironments. Interestingly, the oldest discovered fossilized penis dates to around 100 million years ago. It belongs to an ostracod discovered in Brazil and measures 1 mm (0.4 in.) across. In Arkansas, a report published in 1929 described twenty-six species of ostracods from the Upper Cretaceous (100.5 to 66 million years ago) of the state. Another study reported diverse ostracod assemblages containing a total of forty-nine taxa occurring in the Imo Formation (Mississippian, Chesterian) in north-central Arkansas.
Historically, progress on the study of ostracods was slow and dominated by taxonomy. However, research on fossil ostracods gathered speed in the first half of the nineteenth century. In 1746, Carl von Linné (a.k.a. Carolus Linnaeus, 1707–1778) described an ostracod. The oldest generic names given to ostracods are Cypris and Cythere by the Danish Otto Fredrich Müller (1730‒1784) in the 1770–1780s. The next significant date is 1806, when Frenchman Pierre Andre Latreille (1762–1833) wrote “Subclass Ostracoda Latreille 1806.” In the 1860s, the Norwegian Georg O. Sars (1837‒1937) classified ostracods as an order divided into four suborders: Podocopa, Myodocopa, Cladocopa and Platycopa. The late 1920s and 1930s saw increasing attention paid to ostracods in America and Germany. In 1958, V. Pokórny combined classifications, and, in 1961, an Anglo-American treatise modified Pokórny’s research to establish the foundation of today’s classification system.
Taxonomically, recent molecular and morphological data supports monophyly of the group in the analysis with broadest taxon sampling. Approximately 2,000 ostracod species in 200 genera occur in the non-marine environment. About 1,000 species in 100 genera of those belong to one family, the Cyprididae. Interestingly, many species in this family occupy temporary ponds and have drought-resistant eggs, have mixed/parthenogenetic reproduction, and can swim.
Ostracods range from warm tropical waters to very cold environments such as polar seas. They are also found from intertidal zones to many thousands of meters deep in the sea. Others are also adapted to freshwater niches such as lakes, rivers, and even temporary ponds. In the marine environment, ostracods can be part of the zooplankton as well as considered benthic on the sea floor. Most of the Podocopida inhabit fresh water. There are even terrestrial ostracods, such as the Mesocypris species that lives in humid forest soils in Australia, New Zealand, and South Africa. In addition, ostracods of the family Entocytheridae are obligate ectosymbionts of other crustaceans, including amphipods, crayfishes, isopods, and a species of freshwater crab.
Ostracods vary widely in diet from carnivores to herbivores, scavengers, and filter feeders. Many organisms prey on ostracods, including clams, amphibians, and fishes.
Morphologically, the class is characterized by a body completely enclosed between a hinge with two valves. They have a laterally flattened body. Ostracods have a head and thorax separated by a slight constriction. Unlike those of most other crustaceans, the body is not easily divided into segments. The abdomen of the ostracod is notably regressed or absent. The head possesses the appendages and is the largest part of the body. Two pairs of well-developed antennae are present as well as eight pairs of appendages (though they can be fewer in number), which function mostly in feeding, in locomotion, and for sensory purposes. Typically, ostracods are about 1 mm (0.04 in.) long; however, there is variation in the group, with some members that range from 0.2 to more than 30 mm (0.008 to 1.2 in.). For example, the planktonic genus Gigantocypris can reach 32 mm (1.3 in.) in length.
In terms of physiology, no gills are present, although ostracods retrieve oxygen by branchial plates on the body surface. No heart or genuine circulatory system is present, although blood circulates between the valves of the shell. Nitrogenous wastes are excreted through glands on the maxillae, antennae, or both. Touch is the main sensory sense, although several sensitive hairs occur on the appendages and the body. A single naupliar eye is present, and in some cases they have compound eyes.
Most species reproduce sexually, but some of them reproduce asexually by parthenogenesis. Their reproductive anatomy consists of two penises on the male which correspond to two genital openings or gonopores on the female. Sperm are often large in ostracods and coiled inside of the testes prior to actual mating. Uncoiled, individual sperm may be up to six times longer than the male ostracod himself. Mating occurs during swarming when large numbers of males and females swim together. Eggs are usually placed into the water or onto vegetation or attached to the substrate. In some species, the eggs are brooded inside the shell, thereby protecting them longer. Eggs hatch into a nauplius larva that has a hard shell. Interestingly, entocytherid ostracods are not very discriminate in their choice of mates, as males have been reported to attempt to mate with another male.
Some ostracods, such as Vargula hilgendorfii, possess a light organ in which they produce luminescent chemicals. Most use the light as a defense mechanism, while some others use it for mating (only in the Caribbean). These ostracods are called “blue sand” or “blue tears” and glow blue in the dark.
Ostracods have been reported to cause several problems with fish production ponds such as clogging screens, nets, and equipment due to their small size and large numbers. Another potential problem is that they are an appropriate-sized prey item for walleyes (Sander vitreus), but they do not always get digested and can harm the fish. They may also cause biosecurity concerns when shipping fish or transferring fish from one pond to another.
Various ostracods serve as intermediate hosts for several parasites. The ostracod Physocypria nipponica (Ostracoda: Candonidae) was reported to be an intermediate host of the nematode Anguillicola crassus (Nematoda: Anguillicolidae), a pathogenic swimbladder parasite of eels (Anguilla japonica). Another example is metacercariae of Halipegus occidualis and ostracod second intermediate hosts (Cypridopsis sp.). Interestingly, in that study, reduction in reproductive output, combined with a significantly higher survivorship of exposed ostracods, suggested that infection by H. occidualis may result in ostracod castration. In addition, a new, exceptionally preserved species, described based on adults from 425-million-year-old Silurian age marine rocks, is the only known fossil pentastomid associated with a species of ostracod crustacean. Phosphatized stalked peritrichid ciliates (Ciliophora) have been found attached to ostracods within an Early Triassic ammonoid from Svalbard (Norway). These specimens, however, were not considered true parasites in the strict sense because the specimens were filter feeding.
In Arkansas, there is no comprehensive list of extant ostracods. During the 1804–1805 Hunter-Dunbar Expedition, William Dunbar and his assistant, Dr. George Hunter, noticed a “testaceous bivalve of the size of the minutest grain of sand” in open hot spring pools in Hot Springs (Garland County); this turned out to be an ostracod. Major Stephen Harriman Long’s expedition also recorded ostracods in the thermal waters during its 1818 pass through Hot Springs. Interestingly, two interns at Hot Springs National Park discovered species of ostracods at the former site of the Government Free Bathhouse that had not been previously documented in the park’s thermal waters. Several studies have been published on microbenthic and zooplankton communities that included ostracods collected during various surveys. It is obvious that work on this class is desperately needed in the state.
Cypris is the name of the International Ostracoda Newsletter founded in 1983 by the International Research Group on Ostracoda (IRGO). It collects data from ostracod researchers around the world for annual publication in the newsletter.
For additional information:
Anderson, R. O., and D. Tave. Strategies and Tactics of Fertilized Hatchery Ponds. New York: The Haworth Press, Inc., 1993.
Brandão, S. N., M. V. Angel, I. Karanovic, V. Perrier, and T. Meidla. World Ostracoda Database. Online at http://www.marinespecies.org/ostracoda (accessed November 20, 2020).
Brusca, Robert C., W. Moore, and S. M. Shuster. Invertebrates. 3rd ed. Sunderland, MA: Sinauer Associates, 2016.
Collins, Robert J., Jr. “Stratigraphy and Ostracoda of the Ozan, Annona, and Marlbrook Formations of Southwestern Arkansas.” PhD diss., Louisiana State University, 1960.
Drouant, Ronald G. “Stratigraphy and Ostracoda of the Exogyra costata Zone of Southwestern Arkansas.” PhD diss., Louisiana State University, 1959.
Giere, O. Meiobenthology. The Microscopic Motile Fauna of Aquatic Sediments. 2nd ed. Berlin: Springer-Verlag, 2009.
Grayson, Robert C. “Biostratigraphic and Lithostratigraphic Analysis of the Hindsville Limestone (Mississippian) in Northwestern Arkansas.” Proceedings of the Arkansas Academy of Science 28 (1974): 19‒21. Online at: https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2886&context=jaas (accessed November 20, 2020).
Hoare, R. D. “Ostracodes from the Imo Formation (Mississippian, Chesterian) of North Central Arkansas.” Journal of Paleontology 74 (2010): 1‒23.
“Interns Find Tiny Crustaceans in Arkansas National Park.” Associated Press, August 14, 2017. https://apnews.com/aabab9a708f44761acc2d656ccc548f7 (accessed November 20, 2020).
Israelsky, Merle C. Upper Cretaceous Ostracods of Arkansas. Little Rock: Arkansas Geological Survey, 1929.
Martens, K., and David J. Horne. “Ostracoda.” In Encyclopedia of Inland Waters, edited by G. E. Likens. New York: Elsevier Ltd., 2009.
Martens, K., I. Schon, C. Meisch, and David J. Horne. “Global Diversity of Ostracods (Ostracoda, Crustacea) in Freshwater.” Hydrobiologia 595 (2008): 185–193.
Pitakpaivan, K., and J. E. Hazel. “Ostracods and Chronostratigraphic Position of the Upper Cretaceous Arkadelphia Formation of Arkansas.” Journal of Paleontology 68 (1994): 111‒122.
Pokorný, V. “Contribution to the Morphology and Taxonomy of the Subfamily Hemicytherinae Puri.” Acta Universitatis Carolinae Geologica 2 (1955): 1‒36.
Rickett, John D., and E. B. Floyd. “Survey of the Microbenthic Community in Ferguson Lake, Saline County, Arkansas.” Journal of the Arkansas Academy of Science 55 (2001): 123‒136. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1686&context=jaas (accessed November 20, 2020).
Rickett, John D., and Robert L. Watson. “Zooplankton Community Abundance and Diversity in Dardanelle Reservoir, Arkansas, 1981-1990.” Proceedings of the Arkansas Academy of Science 46 (1992): 57‒60. Online at https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2073&context=jaas (accessed November 20, 2020).
Weaver, Patricia G., and Bronwyn W. Williams. “Observations of False Mating Behavior in Entocytherid Ostracods from the Northwestern United States.” Invertebrate Biology 135 (2016): 252‒258.
Williams, Bronwyn W., Stuart R. Gelder, H. C. Proctor, and D. W. Coltman. “Molecular phylogeny of North American Branchiobdellida (Annelida: Clitellata).” Molecular Phylogenetics and Evolution 66 (2013): 30‒42.
Williams, Bronwyn W., and Patricia G. Weaver. “A Historical Review of the Taxonomy and Classification of Entocytheridae (Crustacea: Ostracoda: Podocopida).” Zootaxa 4448 (2018): 1‒129.
Yamaguchi, S., and K. Endo. “Molecular Phylogeny of Ostracoda (Crustacea) Inferred from 18S Ribosomal DNA Sequences: Implication for its Origin and Diversification.” Marine Biology 143 (2003): 23–38.
Zaharoff, Alexander K., Annie R. Lindgren, Joanna M. Wolfe, and Todd H. Oakley. “Phylotranscriptomics to Bring the Understudied into the Fold: Monophyletic Ostracoda, Fossil Placement, and Pancrustacean Phylogeny.” Molecular Biology and Evolution 30 (2013): 215–233.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology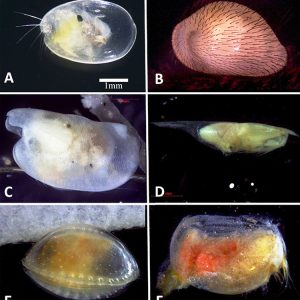 Ostracod Morphology
Ostracod Morphology 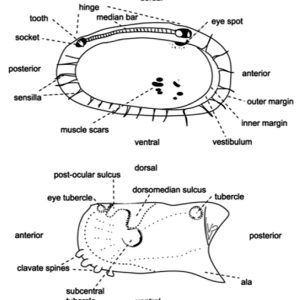 Ostracod Valve Features
Ostracod Valve Features 




Comments
No comments on this entry yet.