calsfoundation@cals.org
Neuropterans
aka: Net-Winged Insects
Neuropterans belong to the Phylum Arthropoda, Class Insecta, and Order Neuroptera. The Neuroptera (antlions, lacewings, mantidflies, and their relatives) and allied orders Megaloptera (dobsonflies, alderflies) and Raphidioptera (snakeflies) together make up the clade Neuropterida. There are three suborders (Osmyloidea, Hemerobiiformia, and Myrmeleontiformia) and nine extinct and fifteen extant families. There are about 6,400 species in the group worldwide, with about 450 found in the United States and Canada. Neuropterans can be found on all continents except Antarctica. There are several types of neuropterans in Arkansas.
In the fossil record, neuropterans first appeared during end of the Permian period (251 million years ago), which ended in the largest mass extinction the Earth ever experienced, as shown by fossils of the Permithonidae from the Tunguska basin in western Siberia and similar fauna from Australia. They continued to flourish and diversify through the Mesozoic era. During the same time, several unusually large forms of neuropterans evolved, especially in the extinct family Kalligrammatidae, which lived from the Jurassic to Aptian (Lower Cretaceous) periods; they are often referred to as “the butterflies of the Jurassic” because they possessed large, patterned wings. The osmylids (giant lacewings) may be the most primal of the Neuropteran groups and are of Jurassic or Early Cretaceous origin. The extinct osmylid, Protosmylus, is fossilized in middle Eocene Baltic amber, and the genus Burmaleon is described from two fossils of Cenomanian-age Burmese amber, implying crown group radiation in the Early Cretaceous or earlier. The family Ithonidae is from the Jurassic to Recent, and the extinct lineages of the family were once geographically widespread. Detailed information from the fossil record has contributed to our understanding of the group’s phylogeny.
Recent molecular analysis of their phylogeny using mitochondrial rRNA and mitogenomic data placed the Neuroptera within the Neuropterida, sister to the Raphidioptera and containing the Megaloptera (sister to the rest of the Neuroptera). The former Neuroptera, particularly the lacewing group, are nonetheless very closely related to each other. Within the endopterygotes, the closest living relatives of the neuropteridan clade are the beetles. However, relationships within the suborder Myrmeleontiformia (antlions, ascalaphids, lacewings, and owlflies) are still in flux.
Neuropterans undergo complete (holometabolic) metamorphosis that includes four very distinct stages: egg, larva, pupa, and adult. Relatively few species have specialized morphological characters, but they still have large lateral compound eyes and may or may not also have simple eyes (ocelli). Their mouthparts possess strong mandibles suitable for chewing but lack the various adaptations found in most other endopterygote insect groups. For example, the larvae of antlions (doodlebugs) and lacewings have specialized mouthparts with maxillae and large, sickle-shaped mandibles that interlock to form pincers. Adult neuropterans have four similarly sized membranous wings, all about the same shape, with a generalized pattern of many veins. Some neuropterans have specialized sense organs in their wings, or have bristles or other structures to link their wings together during flight.
The larval stage of a neuropteran is a specialized predator, with elongated mandibles adapted for piercing and sucking. The larval morphology varies between different families, depending on the nature of their prey. However, in general, they have three pairs of thoracic legs, each ending in two claws. The abdomen often has adhesive discs on the last two segments.
Most families have larvae as omnivorous predators, including many chrysopids (green lacewings and stinkflies), hemerobiids (brown lacewings), and coniopterygids (dustywings). These groups eat aphids (Hemiptera) and other pest insects, and some have been used for biological control (often from commercial distributors, but also abundant and widespread in nature). Larvae in various families cover themselves in debris (sometimes including dead prey insects) as camouflage, radically taken by the ant lions (Myrmeleontidae), which bury themselves completely out of sight and ambush prey from “pits” in the soil. Larvae of some Ithonidae (moth lacewings) are root feeders and will ingest sap from the roots of trees and shrubs. The larvae of Sisyridae (spongillaflies) are aquatic and feed on freshwater sponges, and larvae of most mantidflies (Mantispidae) eat spider egg sacs. Interestingly, adults of many groups are also predatory, but some do not feed, or consume only nectar. However, spoon-winged lacewings (Nemopteridae) and green lacewings eat only pollen and nectar from flowers.
Many adult neuropterans are attracted to lights and active at dusk or in the evening, whereas during the day they remain inactive and hidden among vegetation. Larvae, however, participate in a variety of behaviors to capture prey items. The larvae of owlflies are “sit-and-wait” predators, ambushing prey as they walk into their open jaws. Some antlions construct a trap and hide at the bottom of cone-shaped pits to catch crawling insects. Green lacewings, brown lacewings, and dustywings actively hunt for prey, as do larvae living in freshwater habitats. Some species living along the shore use their long jaws to probe wet sand and mud for fly larvae. Pleasing and silky lacewings hunt in crevices and under bark for arthropods, or animals with hard exoskeletons and several pairs of jointed limbs, such as insects and spiders. The first and third stages of beaded lacewing larvae burrow and hide in the soil in search of termites, while the second stage is inactive and does not feed.
For communication, many male neuropterans have special organs on their abdomen or wings that produce pheromones to be in touch with potential mates during courtship. Green lacewing males use sound to attract mates, vibrating their abdomens to communicate with females.
Males deposit sperm directly into the genitalia of the female. Mating is either brief or lasts up to several hours. Females lay eggs one at a time or in batches on rocks, bark, or in crevices of bark. Some species of green lacewings, mantidflies, and split-footed lacewings lay a single egg on top of a silk stalk to keep them out of the reach of predators, especially other lacewing larvae. Eggs will develop into larvae, and there is little or no parental care of the eggs. The larvae look nothing like the adults and do not live in the same habitat. The larvae shed their exoskeletons three times over several months or years before transforming into pupae. During development, the pupal stage is enclosed in some kind of cocoon composed of silk and soil or other debris. The pupa eventually eats its way out of the cocoon with its mandibles and may even move about for a short while before undergoing the molt to the adult form. Neuropteran larvae produce silk with special organs inside their abdomen. The legs and wings of the pupa are not completely attached to the body, and the abdomen is capable of some movement.
Predators of neuropteran larvae include some beetles (Coleoptera), wasps (Hymenoptera), and some dipteran chironomids (nonbiting midges, lake flies) that parasitize them. Some neuropterans rely on camouflage to evade detection by predators. Some brown and green lacewings will feign death when threatened. Others produce an offensive odor to discourage predation. Some mantidflies not only mimic the color and appearance of paper wasps but will also adopt their movements and postures when disturbed.
In terms of neuropterans and humans, the larvae of green and brown lacewings are known as aphidlions, and they prey on pests in a variety of garden, greenhouse, and agricultural settings. They are also sold to farmers and gardeners as eggs, and the adults are reared by the thousands and released among various crops to control pests (insects and mites). However, research on the use of neuropterans in the biological control of insect pests has shown that it is difficult to establish and maintain populations in fields of crops.
Five species of neuropterans are also among the 1,681 insect species eaten by humans worldwide. In Papua New Guinea, the Highland people claim to be able to maintain a muscular build and great stamina despite their low protein intake as a result of eating insects such as neuropterans.
In Arkansas, several species of neuropterans have been reported from the Ouachitas and Ozarks, but additional surveys are needed outside the Interior Highlands. Unidentified green (Chrysopidae) and brown lacewings (Hemerobiidae) have been reported from malaise traps set in northeastern Arkansas. The spongillafly Climacia areolaris has been reported from Clark, Garland, Johnson, Lafayette, Logan, Ouachita, Perry, Pike, Pope, and Stone counties. Another, Sisyra vicaria, has been found in Logan and Poinsett counties. It is a univoltine (producing one brood in a season) species and overwinters in terrestrial eggs as pharate first instars. Interestingly, S. vicaria appears after the first generation of C. areolaris.
For additional information:
Arnett, Ross H. American Insects: A Handbook of the Insects of America North of Mexico. Boca Raton: CRC Press, 2000.
Bowles, David E. “New Distributional Records for Neotropical Spongillaflies (Neuroptera: Sisyridae).” Insecta Mundi 0400 (2015): 1‒7.
———. “Spongillaflies (Neuroptera: Sisyridae) of North America with a Key to the Larvae and Adults.” Zootaxa 1357 (2006): 1‒19.
Bowles, David E., Atilano Contreras-Ramos, M. Sarmiento, and M. L. Ferro. “New Distributional Records for Pleasing Lacewings (Neuroptera: Dilaridae, Nallachius spp.) in the Americas.” Insecta Mundi 0406 (2015): 1‒10.
Brown, Harley P. “Distributional Records of Spongilla Flies (Neuroptera: Sisyridae).” Entomological News 85 (1974): 31–33.
–——. “The Life History of Climacia areolaris (Hagen), a Neuropterous ‘Parasite’ of Freshwater Sponges.” American Midland Naturalist 47 (1952): 130–160.
———. “Observations on Spongilla fragilis: Reorganization and Larvae.” Proceedings of the Oklahoma Academy of Science 30 (1949): 30–32.
Cockerell, T. D. A. “Fossil Osmylidae (Neuroptera) in America.” Canadian Entomologist 40 (1908): 341–342.
Cooksey, Lynita M., and Harvey E. Barton. “Flying Insect Populations as Sampled by Malaise Trap on Crowley’s Ridge in Northeast Arkansas.” Proceedings of the Arkansas Academy of Science 35 (1981): 29‒32. Online at https://scholarworks.uark.edu/jaas/vol35/iss1/9 (accessed September 16, 2021).
Engel, Michael S. “A Remarkable Kalligrammatid Lacewing from the Upper Jurassic of Kazakhstan (Neuroptera: Kalligrammatidae).” Transactions of the Kansas Academy of Science 108 (2005): 59–62.
Engel, Michael S., and David A. Grimaldi. “The Neuropterid Fauna of Dominican and Mexican Amber (Neuropterida, Megaloptera, Neuroptera).” American Museum Novitates (2007): 1–58.
Haring, E., and U. Aspöck. “Phylogeny of the Neuropterida: A First Molecular Approach.” Systematic Entomology 29 (2004): 415–430.
Jones, Joshua R. “Total‐Evidence Phylogeny of the Owlflies (Neuroptera, Ascalaphidae) Supports a New Higher‐Level Classification.” Zoologica Scripta (2019): https://doi.org/10.1111/zsc.12382 (accessed September 16, 2021).
Penny, Norman D., Phillip A. Adans, and Lionel A. Stange. “Species Catalog of the Neuroptera, Megaloptera and Raphidioptera of America North of Mexico.” Proceedings of the California Academy of Sciences, Fourth Series 50 (1997): 39–114.
Poirrier, M. A. “Some Freshwater Sponge Hosts of Louisiana and Texas Spongilla-Flies, with New Locality Records.” American Midland Naturalist 81 (1969): 573–575.
Reynoso-Velasco, Daniel, and Atilano Contreras-Ramos. “Taxonomic Review of the Mantidfly Genus Nolima Navás (Neuroptera, Mantispidae, Calomantispinae).” Zookeys 853 (2019): 131‒158.
Song, Nan, Xin-Xin Li, Qing Zhai, Hakan Bozdoğan, and Xin-Ming Yin. “The Mitochondrial Genomes of Neuropteridan Insects and Implications for the Phylogeny of Neuroptera.” Genes 10 (2019): 108.
Wang, Y., X. Liu, and I. J. Garzón‐Orduña, et al. “Mitogenomic Phylogeny Illuminates the Evolutionary History of Lacewings (Insecta: Neuroptera).” Cladistics 33 (2016): 1–20.
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology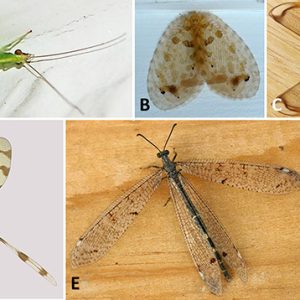 Neuropterans
Neuropterans 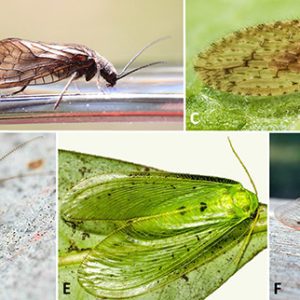 Neuropterans
Neuropterans 




Comments
No comments on this entry yet.