calsfoundation@cals.org
Dog Heartworms
aka: Dirofilaria immitis
The canine heartworm (Dirofilaria immitis) is a filarial parasite that belongs to the Phylum Nematoda, Class Secertenea, Order Spirurida, and Family Onchocercidae. There are two subgenera: Dirofilaria and Nochtiella. This parasite is often found in wild and domestic canids throughout the world, especially in the United States where it is endemic from the East to the Midwest, the southeastern Atlantic seaboard, and the southern Gulf Coast. Transmission of the parasite occurs throughout the United States (even Alaska) and in the warmer regions of Canada. In the United States, the highest infection rates are found within 241 km (150 mi.) of the coast from Texas northeast to New Jersey, and along the Mississippi River Valley and its major tributaries. The parasite has also been reported from South America, southern Europe, Australia, Southeast Asia, Korea, Japan, and the Middle East. Dirofilaria immitis is found in many tropical, subtropical, and temperate regions, particularly in humid areas and river valleys where environmental conditions harbor breeding mosquito vectors. Arkansas is one of the top five states in the United States for incidence of heartworm infection.
In 1586, Chez Iean Wolfe made a sketch of a “monster” found inside the heart of a horse, which was later identified as D. immitis. A nobleman named Francesco Birago made the first known reference to canine infection of D. immitis in the seventeenth century by describing the presence of adult worms inside the hearts of his hunting dogs, although he erroneously identified them as larvae of another parasitic worm. In the United States, canine heartworms were first discovered in 1856 on the southeastern coast. The parasite was formally described in 1856 by Joseph Mellick Leidy (1823‒1891). It was not until the 1920s that an infection by the same organism was discovered in cats. However, the first human infection was not reported until 1952 in America.
Transmission of this zoonotic disease (dirofilariasis) is by vectors including several species of mosquitoes in the genera Aedes, Anopheles, and Culex. Felids can also be infected with this nematode but appear to harbor a smaller intensity of adult worms. Unlike the infection in dogs, the vast majority of larval heartworms do not survive in cats. Other definitive hosts include coyotes, foxes, wolves, sea lions, harbor seals, bears, laboratory ferrets, horses, beavers, muskrats, raccoons, red pandas, leopards, and wolverines.
Adult D. immitis inhabit the pulmonary arteries, and during severe infections can migrate into the right atrium, right ventricle, and inferior vena cava leaving the heart. Large worms can sometimes extend through the openings of the tricuspid and pulmonary semilunar valves. The larvae, called microfilariae, are found in the final host’s blood. Although D. immitis has been reported from humans, its larvae do not mature into adults.
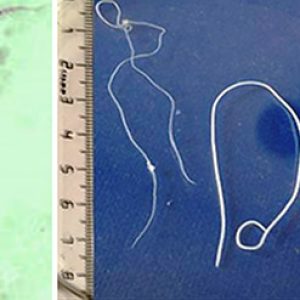
Morphologically, adult D. immitis are cylindrical, slender white worms, with adult males measuring 12 to 16 cm (4.7 to 6.3 in.), smaller than the adult female, which is 25 to 30 cm (9.8 to 11.8 in.). As a roundworm member, it has a cuticle with three main outer layers made of collagen and other compounds. The outer layers are acellular and are secreted by the epidermis. They also have longitudinal muscles along the body wall in obliquely arranged bands. The dorsal, ventral, and longitudinal nerve cords are connected to the main body of the muscle. The male’s caudal end is spirally coiled and its tail has many alae (thickenings of the cuticle) whereas the posterior end of the female is straight. Both sexes have a mouth, a filariform esophagus, a nerve ring, and excretory and anal pores. The male has a seminal vesicle and testis while the female possesses an ovary and oviduct. The microfilariae are about 307 to 322 µm long and 6.7 to 7.1 µm wide.
The microfilariae of D. immitis can be confused morphologically with another filariid, the nonpathogenic nematode, Dipetalonema reconditum. This cosmopolitan nematode is commonly endemic in dogs’ subcutaneous tissues, including canids in the United States, Italy, and Africa. Its infection in dogs is not clinically significant, although hosts may have a noticeable elevated white blood cell (leucocyte) count, particularly a high eosinophil count. This hematological symptom may result in false positive tests for circulating D. immitis microfilariae. To help differentiate these two worms, Knott’s test is used to demonstrate these microfilariae serologically by their staining patterns with acid phosphatase as follows: Dipetalonema stains evenly while Dirofilaria concentrates the acid phosphatase in two regions. A more specific differentiation can be done using PCR (polymerase chain reaction). There are a few reports of D. reconditum in humans. Because it is a self-limiting condition, the definitive treatment is surgical excision of the worm.
Reproduction in D. immitis involves a pheromone produced by females to entice males. The male worm coils around a female, with its curved region over the genital pore of the female. The male gubernaculum, made of sclerotized cuticular tissue, guides the spicules that extend through the cloaca and anus to hold on to the female during copulation. Amoeboid-like sperm is deposited by the male and are without flagella.
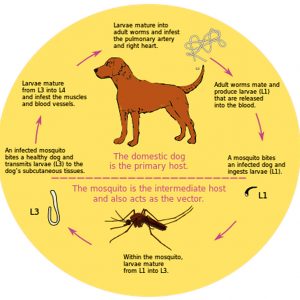
In the complex life cycle of D. immitis, the mosquito vector ingests microfilariae (first stage juvenile larva or L1) after a blood meal from an infected dog or another host. The L1 develops into second stage larvae (L2) and feeds on cells in the malpighian tubules (renal excretory system) of the mosquito vector. Next, the third-stage larvae (L3) develop and enter the body cavity (hemocoel) of the mosquito vector where development takes fifteen to sixteen days. The L3 escape in a pool of hemolymph (analogous to blood in vertebrates) as the vector inserts its labium (outer proboscis sheath) and fascicle into the flesh of a definitive host, which is usually a dog. Once its stylets (mouthparts that actually pierce and enter the skin) are withdrawn, the L3 enter through the puncture wound. The L3 then molts into the fourth larval stage (L4) from zero to fourteen days after infection in the definitive host. The L4 remain dormant after migrating to submuscular membranes and subcutaneous tissue. They molt into the fifth larval stage (L5) and further migrate through the walls of venules, where they end up in the pulmonary arterioles and right side of the heart in the definitive host. After 85 to 120 days, the L5 will mature into adults that will further migrate to the host’s right ventricle or pulmonary artery days after infection. The adult will reach maturity in a further sixty-day period and shed microfilariae in the blood to begin the life cycle all over again. The adult worms can remain in the heart or pulmonary artery for up to seven years, whereas the microfilariae can remain in the circulation of the mosquito for up to two years.
Fortunately for humans, the nematodes cannot mature into the adult stage and cease development in larval stages. Therefore, no microfilariae are ever present in human blood, since the parasites can never fully develop to shed the microfilariae into the blood. However, human infections should be not taken lightly, as the parasite can cause pulmonary dirofilariosis if immature worms begin developing in nodules in the lungs or other subcutaneous tissues. Rarely, worms have been found in humans outside the lungs, including in the brain, eye, and testicle. Also in humans, the clinical symptoms are less noticeable non-specific signs, including cough, hemoptysis (coughing up blood), lack of stamina, malaise, chest pain, and wheezing. Interestingly, although heartworms are helminth parasites that typically cause hosts to have peripheral eosinophilia, that specific white blood cell increase is only found in about 6.5 to 15 percent of the cases. A review of cases of U.S. human dirofilariasis published in 2005 lists a total of eighty-one instances reported in the literature since 1941, a span of more than six decades.
This dog heartworm is of utmost veterinary importance because it constitutes a potentially dangerous pathogen to dogs and cats. In heavy infections of these hosts, the worms can cause circulatory distress, including interference with heart valve functionality and congestion of the right side of the heart. In addition, if no treatment is administered, cirrhosis of the liver and endarteritis (inflammation of the intima of an artery) can become apparent after nine to ten months. Initial clinical symptoms in dogs show signs of chronic coughing, decreased exercise endurance, and collapse after exercising.
Diagnosis of heartworm infection includes testing the blood of suspected hosts for microfilariae and performing thoracic radiography, electrocardiogram, blood chemistry, and urinalysis. More often in the past, microscopic identification of microfilariae on a direct blood smear using the modified Knott technique, or after millipore filtration, was conducted. The former technique is not recommended as a catch-all diagnostic test for D. immitis because infections may consist of male worms that do not produce microfilariae, or immature female worms that are not yet producing microfilariae. The accuracy of these tests, typically used for routine screening or diagnosis of heartworm infection, is improved by multiple testing. Also in clinical practice, determining heartworm infection relies upon detecting antigen of D. immitis in serum, plasma, or whole blood samples from canine and feline patients. Traditional microfilarial testing was also widely used to test dogs; however, in most canine surveys sensitive antigen assays is superior in detecting more infections than tests for microfilariae. The majority of surveys of naturally infected dogs that include both approaches provide evidence that the antigen detection identifies more heartworm infections. Even more surprising is that in some populations, a good number of samples are antigen-negative but microfilaria-positive. To help avoid discordant results, heat pretreatment of serum or plasma samples offers a valuable accessory to traditional heartworm testing. The guidelines of the American Heartworm Society state that the current generation of heartworm antigen tests are “nearly 100% specific” and the label information on the assays and data from available comparative studies lend support to this assertion. The gold standard diagnostic tools for potential human infections are lung biopsy and chest x-ray.
It is very important to keep dogs indoors at night during peak mosquito biting hours. Also, spraying to lessen mosquitoes as well as keeping standing water outdoors at a minimum would help control the disease by decreasing the vector population. Dogs living in open air and meager conditions seem to be more prone to becoming infected. The adaptability of some vector mosquito species, particularly those in the genus Culex, plays an important role in the spread of infection.
Preventative techniques administered by regular veterinary visits can drastically reduce the risk of infection in domestic animals. For example, pills or tablets of chewable ivermectin/pyrantel, milbemycin, and moxidectin are used to kill the larvae and prevent additional infections. Selamectin is a liquid applied topically on a dog or cat that safely prevents the transmission of the adult heartworm; it is not approved for use in humans. Another preventative, Trifexis (spinosad and milbemycin oxime), is a monthly, beef-flavored tablet that inhibits heartworm disease. However, treatment with fewer than three monthly doses after the last exposure to mosquitoes may not provide complete heartworm prevention. All of these preventative drugs are highly effective when regularly administered and protect more than ninety-nine percent of dogs and cats from heartworm. As early as eight weeks old, heartworm prevention can be started in dogs. The topical or oral preventatives can be administered monthly or every six months, and a continuous twelve-month prevention schedule is recommended.
Today, due to resistant strains of heartworms circulating in the southern United States, against which no preventative can protect, the American Heartworm Society recommends dogs be on a combined regimen of repellent in addition to a heartworm preventative. The state of Louisiana hosts by far the most cases of suspected drug-resistant heartworm strains.
Treating a dog already diagnosed with heartworm disease is not an easy task. Before the dog or another host can be treated, it must be evaluated for heart, liver, and kidney function to evaluate the risks and benefits of treatment. If those check out, initial treatment may begin with a course of antibiotics (doxycycline), heartworm preventives, and steroids given before the actual adult worm treatment. Since the heartworm treatment only kills adult worms, a monthly heartworm preventive designed to kill the smaller larvae might be given before initiating adult heartworm treatment. Administration of corticosteroids at the same time as the antibiotics and heartworm preventive also helps reduce inflammation, a common side effect. Once a dog has completed the course of steroids, heartworm preventives, and antibiotics, it should be ready to start the actual adult heartworm treatment. The treatment for heartworm disease takes at least sixty days to complete and consists of a series of drug injections that kills the worms. Unfortunately, melarsomine is the only drug approved to kill adult heartworms in dogs, and it is an organic arsenical compound that is injected into the dog’s lumbar muscles. Treatment entails an extended stay for observation and limited activity for months thereafter. Dogs that remain heartworm positive six months after treatment may need to repeat treatment to kill the remaining worms. As a last resort, surgical removal of the adult heartworms as a treatment also may be indicated, especially in advanced cases with substantial heart involvement.
Besides D. immitis, there are about twenty-six species of Dirofilaria. Of these, two important species include D. (Nochtiella) repens and D. tenuis. The former is also found in dogs and cats and other carnivores such as foxes, coyotes, wolves, and sea lions, as well as muskrats in the Mediterranean region, sub-Saharan Africa, Eastern Europe, and South Asia. Like D. immitis, it is transmitted by mosquitoes.
According to the American Heartworm Society, in 2016, the top five states in heartworm incidence were, in order: Mississippi, Louisiana, Arkansas, Texas, and Tennessee. Rounding out the top ten states were South Carolina, Georgia, North Carolina, Alabama, and Florida. In one study of potential mosquito vectors in northeastern Arkansas, D. immitis was identified in nine species, including Aedes vexans, Anopheles quadrimaculatus, An. punctipennis, Culex pipiens, quinquefasciatus, C. erraticus, Culiseta inornata, Psorophora columbiae, P. ferox, and P. howardii. Another study from Arkansas reported a case of D. immitis in the eye of a dog from Pulaski County. In addition, the first report of human infection of D. immitis in Arkansas was documented in 2004 when a resident presented with a solitary pulmonary nodule that pathologic examination revealed to be a granuloma secondary to dirofilariasis.
Improved instructive diagnostic tests for dirofilariasis have led scientists to explore the possibility that new methodologies now used in other diseases, such as using molecular markers, may be more valuable than the present-day immunological assays. The use of microRNAs (miRNAs) has been suggested as the basis for a new generation of diagnostic tests that can provide important information for veterinary medicine, and this approach may assist in the development of optimal treatment regimens for this common insidious parasite.
For additional information:
Bowman, D. D., and C. E. Atkins. “Heartworm Biology, Treatment, and Control.” Veterinary Clinics Small Animal Practice 39 (2009): 1127‒1158.
Cima, G. “Heartworm Preventive Resistance Is Real.” Journal of the American Veterinary Medical Association 243 (2013): 1230.
Eberhard, Mark L., James J. Daly, Susan Weinstein, and Harold E. Farris. “Dirofilaria immitis from the Eye of a Dog in Arkansas.” Journal of Parasitology 63 (1977): 978.
Foreyt, William J. Veterinary Parasitology: Reference Manual. Ames: Iowa State University Press, 2001.
Hoch, H., and K. Strickland. “Canine and Feline Dirofilariasis: Life Cycle, Pathophysiology, and Diagnosis.” Compendium of Continuing Education Veterinary Medicine 30 (2009): 133‒140.
Jacobs, D. E. Principles of Veterinary Parasitology. Hoboken, NJ: John Wiley & Sons, Ltd., 2016.
Jones, J. W., M. V. Meisch, and F. L. Farmer. “Survey of Dirofilariasis in Arkansas.” Journal of the American Mosquito Control Association 9 (1993): 235–237.
Lee, A. C. Y., S. P. Montgomery, J. H. Theis, Byron L. Blagburn, and M. L. Eberhard. “Public Health Issues Concerning the Widespread Distribution of Canine Heartworm Disease.” Trends in Parasitology 26 (2010): 168–173.
Levine, Norman D. Nematode Parasites of Domestic Animals and Man. 2nd ed. Minneapolis: Burgess Publishing Company, 1980.
McCall, J. W., C. Genchi, L. H. Kramer, J. Guerrero, and L. Venco. “Heartworm Infection in Animals and Humans.” Advances in Parasitology 66 (2008): 193‒285.
McKay, Tanya, T. Bianco, L. Rhodes, and S. Barnett. “Prevalence of Dirofilaria immitis (Nematoda: Filarioidea) in Mosquitoes from Northeast Arkansas, the United States.” Journal of Medical Entomology 50 (2013): 871‒878.
Mumtaz, H., A. Ozdemir, and R. C. Schaefer. “Case of the Month. A Case Report of Human Pulmonary Dirofilariasis in Arkansas.” Journal of the Arkansas Medical Society 100 (2004): 240‒242.
Patton, Sharon, and Malcolm D. McCracken. “Prevalence of Dirofilaria immitis in Cats and Dogs in Eastern Tennessee.” Journal of Veterinary Diagnosis and Investigation 3 (1991): 79–80.
Pistey, Warren R. “Studies on the Development of Dirofilaria tenuis Chandler 1942.” Journal of Parasitology 44 (1958): 613–626.
Sawyer, Thomas K., and Paul P. Weinstein. “Studies on Microfilariae of Dog Heartworm Dirofilaria immitis: Separation of Parasites from Whole Blood.” Journal of Parasitology 49 (1963): 39–45.
Taylor, Michael L., Richard L. Coop, and Richard L. Wall. Veterinary Parasitology. 4th Ed. Hoboken, NJ: Wiley-Blackwell, 2016.
Theis, J. H. “Public Health Aspects of Dirofilariasis in the United States.” Veterinary Parasitology 133 (2004): 157‒180.
Underwood, Paul C., and Paul D. Harwood. “Survival and Location of the Microfilariae of Dirofilaria immitis in the Dog.” Journal of Parasitology 25 (1939): 23–33.
Velasquez, L., Byron L. Blagburn, R. Duncan-Decoq, E. M. Johnson, K. E. Allen, and J. Meinkoth et al. “Increased Prevalence of Dirofilaria immitis Antigen in Canine Samples After Heat Treatment.” Veterinary Parasitology 206 (2014): 67–70.
Wang, D., D. D. Bowman, H. Brown, L. C. Harrington, P. E. Kaufman, Tanya McKay, C. T. Nelson, J. L. Sharp and R. Lund. “Factors Influencing U.S. Canine Heartworm (Dirofilaria immitis) Prevalence.” Parasites & Vectors 7 (2014): 264.
Chris T. McAllister
Eastern Oklahoma State College





Comments
No comments on this entry yet.