calsfoundation@cals.org
Apicomplexans
aka: Sporozoans
The protistan Phylum Apicomplexa (formerly Sporozoa) contains a tremendous variety of obligate intracellular parasites infecting many different organisms, including humans. As a group, these parasites are cosmopolitan in their range of infected hosts and geographic distribution. They include such diverse parasites as coccidians, cryptosporids, gregarines, haemosporoids, and piroplasms. All are united, not by their biology or life histories, but morphologically by the presence of a unique structure called an apical complex. The classification scheme that cites this structure has a practical purpose to sort this diversity in a functional manner that can: (1) be easily understood and, (2) serve a utilitarian purpose by non-specialists. However, the field of classifying Apicomplexa is in flux; indeed, its taxonomy has changed throughout the years since it was formally named by Norman D. Levine in 1970.
The study of apicomplexans in Arkansas has become increasingly popular, although several significant groups have not yet been studied. Descriptions have increased, however, of many new and previously described species of coccidians from various animals surveyed in the state such as amphibians, reptiles, and mammals.
Historically, the first apicomplexan was viewed by father of protistology and the microscope Antonie Philips van Leeuwenhoek (1632‒1723), who, in 1674, probably saw oocysts of the rabbit coccidian, Eimeria stiedae. The initial species to be described was a gregarine, Gregarina ovata, by Dufour in 1828 from the intestine of earwigs, Folficula aricularia (Dermaptera). Since then, many more have been identified and formally described. The largest subgroup of the phylum is the suborder Eimeriorina, which contains organisms collectively referred to as the coccidia. The Eimeriorina has eight to thirteen families, thirty-six to thirty-nine genera, and more than 2,000 named species and growing. By far the largest family in the suborder, Eimeriidae, houses at least seventeen genera and about 1,700 species. Although most coccidians have been reported from various vertebrates ranging from fishes to mammals, a few have been reported from invertebrates such as millipedes, centipedes, and snails.
Evolutionarily, all apicomplexans are parasitic and evolved from a free-living ancestor. This natural history is presumed to have evolved at the time of the divergence of dinoflagellates (flagellated unicellular eukaryotes) and apicomplexans. Additional evolution of this phylum has been estimated to have occurred about 800 million years ago when a global glaciation may have occurred. The archigregarines (found only in marine habitats) are thought to be the oldest extant clade.
All apicomplexans have present at some stage in their life cycle a single type of plastid-like organelle called an apicoplast. The function of this apical organelle complex is to penetrate a host cell. In addition, there are micropore(s) (cytostome), micronemes, flattened subpellicular vesicles, a conoid, one or more polar rings, rhoptries, and subpellicular microtubules. Apicomplexans have typical eukaryotic organelles such as a nucleus, an endoplasmic reticulum, a single mitochondrion, and a Golgi apparatus. However, cilia and flagella (except for flagellated microgametes), centrioles, chloroplasts, ejectile organelles, and cytoplasmic inclusions are absent. Most members have a complex life cycle, involving both asexual and sexual reproduction, and it appears to be haploid except for a brief diplophase when zygotes are formed.
This composite of protists is subdivided into two major assemblages based on the presence or absence of a conoid in their apical complex. The first major grouping, Aconoidasida, all lack a conoid in their asexual motile stages and are one-host parasites of invertebrates or vertebrates, or two-host parasites that alternately infect haematophagous (blood-feeding) invertebrates and the blood of vertebrates. They include the Haemospororida with about 500 species within four families (Garniidae, Haemoproteidae, Leucocytozoidae, Plasmodiidae) and containing about nineteen genera (Akiba, Bioccala, Biguetiella, Billbraya, Dionisia, Fallisia, Garnia, Haemocystidium, Haemoproteus, Hepatocystis, Hepatozoon, Johnsprentia, Leucocytozoon, Mesnilium, Nycteria, Paleohaemoproteus, Plasmodium, Progarnia, and Sprattiella) and Piroplasmorida with four families (Babesiidae, Dactylosomatidae, Theileriidae) containing eight genera (Babesia, Dactylosoma, Echinozoon, Entopolypoides, Haemohormidium, Haematoxenus, Sauroplasma, and Theileria). The most important to human health are the malarial parasites, which are two-host apicomplexans that parasitize blood-feeding dipteran flies (female mosquitoes) and the blood of various tetrapod vertebrates. In addition, piroplasms possess all species that are two-host parasites infecting ticks and vertebrates.
The second major grouping, Conoidasida, all have a complete apical complex that includes a hollow, truncated conoid in all or most of their asexual motile stages, along with other unifying morphological features. This paraphyletic lineage includes three groups: (1) a diverse group called Coccidia that includes one host species of invertebrates, two-host species of invertebrates, one-host species of vertebrates, and two-host species of vertebrates, (2) Gregarinasina, and (3) the monogeneric family Cryptosporidiidae. Interestingly, parasitologists are beginning to realize that Cryptosporidium species, once considered to be “atypical” Coccidia, are most closely related to the gregarines and not to the Coccidia.
Coccidians all possess mature gametes that develop intracellularly, microgametocytes that usually produce many microgametes, and non-motile zygotes that mainly contain sporocysts within their oocysts. There are two Coccidia lineages: Adelorina and Eimeriorina. The former includes about seven families (Adeleidae, Dactylosomatidae, Haemogregarinidae, Hepatozoidae, Karyolysidae, Klossiellidae, and Legerellidae), two of which contain a genus of parasites recorded from a variety of vertebrates (such as turtles, crocodiles, birds, other reptiles, and mammals), Hepatozoidae (Hepatozoon), and Haemogregarinidae (Haemogregarina). The classification of these two genera has undergone significant revision, and it is important to list the key features that distinguish one from another. The genus Haemogregarina was once a taxonomic catch-all for virtually all red blood cell (rbc) parasites of birds, reptiles (particularly turtles), and crocodilians, and usually named as novel or unique when found in a vertebrate from which no others were described or surveyed previously. Often, most of these poorly described forms were transferred to Hepatozoon once more information became available. Species in both genera are heteroxenous, with gamonts found in the erythrocytes or rbc’s or leucocytes (white blood cells, wbc’s) of the vertebrate intermediate host. Sexual conjugation, via syzygy, is followed by sporogony, both of which occur in the invertebrate definitive host. Species of Haemogregarina possess gametes that mature inside a leech’s gut (the only complete life cycle known), and oocysts then develop in the epithelial cells of the leech’s gut. Here, they contain only “naked” sporozoites without sporocysts. These sporozoites migrate to the salivary glands of the leech, which, when it takes another blood meal, then infects the next vertebrate host.
In Hepatozoon species, many arthropods can serve as definitive hosts, including anopheline and culicine mosquitoes (in their malpighnian tubules), sandflies, ticks, tsetse flies, and others. After their blood meal, gamonts undergo syzygy and gametogenesis, and sporogonic development in the arthropod results in the formation of large polysporic oocysts usually with thick walls and found in the gut wall or hemocoel of the arthropod. Transmission occurs only when the infected arthropod is ingested by the vertebrate host. Both genera are similar in that once infected by the invertebrate host, pre-erythrocyte merogony occurs in the vertebrate prior to the invasion of blood cells by the merozoites to form gametes. A good example in this family is H. catesbianae, a parasite of the American bullfrog (Rana catesbeiana) and the mosquito, Culex territans. After ingestion by the frog, and after one round of asexual development in the frog’s liver, parasites emerge and infect rbc’s, where they form gamonts. Lesser genera in this group include Babesiosoma, Bartazoon, Cyrilia, Dactylosoma, Desseria, Hemolivia, and Karyolysus.
The largest subgroup of the phylum is the suborder Eimeriorina, which contains the Coccidia. They are predominantly intestinal parasites that are capable of infecting most phyla of invertebrates and all vertebrate classes. The disease they generally cause, coccidiosis, has been recognized as a major health hazard in domestic animal husbandry, in zoological park environments, and in wild animal populations when habitat dwindles and overcrowding occurs. One coccidian, Eimeria tenella, is responsible for considerable decrease in growth and development of domestic poultry (primarily chicken) flocks by damage caused by infection of the cecal mucosa. This costs the industry more than $1.5 billion annually. The Eimeriorina comprises a variety of “typical” coccidians, many of which form cysts. A number of genera—including Caryospora, Cyclospora, Cystoisopora, Eimeria, Isospora, Sarcocystis, and Toxoplasma—infect vertebrates. All coccidians in birds (Aves) of the genus Caryospora are now referred to a new genus, Avispora.
The gregarines (Gregarinasina) are generally one-host parasites that infect the gut (and other tissues) of invertebrates (primarily annelids, arthropods, and mollusks). They were recognized as a separate taxon in 1953 by Pierre-Paul Grassé (1895‒1985). About 250 genera and 1,650 species are known in this taxon. Although closely related to the malarial parasites of humans, gregarines do not infect vertebrates. Some common examples include Monocystis lumbrici, which inhabits the seminal vesicle of the common earthworm (Lumbricus terrestris) and other related earthworms. Another common gregarine is Gregarina cuneata, a parasite of the beetle mealworm (Tenebrio molitor), found especially in colonies of beetles in laboratory colonies. Although several gregarine genera have been reported from cockroaches, two genera comprising the family Blabericolidae are associated exclusively with cockroach hosts. This family includes the genera Blabericola and Protomagalhaensia. Interestingly, although some cockroach species host only a single gregarine, among tropical cockroach species it is common to find pairs of gregarine species infecting the same host species. In all cases examined to date, these gregarine pairs include a single species of Protomagalhaensia and one species of Blabericola. As all gregarine species known from cockroaches thus far have been found to be host specific, this example is a good model for studying co-evolutionary processes. Other gregarines have been found in some damselflies and dragonflies, polychaetes, crustaceans, and myriapods.
As a whole, the phylum is a diverse group that includes organisms that cause human diseases such as babesiosis (Babesia), cryptosporiidiosis (Cryptosporidium parvum), cyclosporiasis (Cyclospora cayetanensis), cystoisosporiasis (Cystoisospora belli), malaria (Plasmodium), and toxoplasmosis (Toxoplasma gondii).
In 1893, it was first reported that ticks transmit the piroplasm Babesia bigemina. The disease it causes, babesiosis, is a malaria-like disease caused by erythrocytic infection with this parasite. The disease in humans is most commonly observed in people in the northeastern and midwestern United States and parts of Europe; it is sporadic throughout the remainder of the world. Babesia microti is typically transmitted by the bite of infected nymphal Ixodes scapularis tick. In addition to getting the disease by the more common route above, humans can also acquire it by getting a blood transfusion from an infected donor, or by congenital transmission (from an infected mother to her infant). Babesia can also have a major impact on the health of domestic animals in areas without cold winters, such as in domestic cattle (Bos taurus) when the disease is known as piroplasmosis, redwater, or Texas cattle fever.
Another tickborne piroplasm, Theileria species, infects and causes disease in livestock, and especially cattle, in many parts of the globe. The most serious disease is East Coast fever of cattle, caused by T. parva, which has 90 to 100 percent mortality in Africa. Another species, T. annulata, causes a milder disease of cattle along the Mediterranean and in the Middle East known as tropical theileriosis.
Cryptosporiidiosis is a serious emerging human pathogenic disease. Since 1976, it has been a debilitating illness causing enteritis in humans, and various Cryptosporidium species infect other animals (primarily mammals) causing a waterborne diarrheal disease. Ingestion of infective oocysts leads to the disease, which may range from asymptomatic to relatively mild cases of diarrhea in immunocompetent individuals to potentially life-threatening profuse watery diarrhea in patients who have weakened immune systems, such as those with AIDS. There are no drugs (coccidostats) that completely get rid of the disease, but some, like Alinia (nitazoxanide), may help slow down the progression. In hosts with healthy immune systems, the disease is self-limiting in a few days.
Another pathogenic apicomplexan of humans (perhaps also in other primates as well) is Cyclospora cayetanensis. The first human cases of C. cayetanensis were reported in 1979, but it was not fully recognized until the early 1990s, and is most common in tropical to subtropical regions. Infection leads to a gastrointestinal disease, usually from foodborne outbreaks, called cyclosporiasis that can range from asymptomatic to debilitating. Restaurant-associated cases have been reported in the United States, linked to salad mixes that contained contaminated iceberg and romaine lettuce, red cabbage, carrots, and cilantro. Most people who have healthy immune systems will recover without treatment, although the illness may last for a few days to a month or longer. Trimethoprim/sulfamethoxazole (Bactrim) is the usual therapy for Cyclospora infection. To date, no highly effective alternative antibiotic regimen has been identified for patients who do not respond to the standard treatment or have an allergy to sulfa drugs.
Cystoisosporiasis is an intestinal disease of humans caused by the coccidian parasite Cystoisospora belli (formerly Isospora belli). It is most common in tropical and subtropical areas of the planet. The parasite can be spread by ingesting contaminated food or water. In immune-competent people, C. belli infection is often asymptomatic. The most common symptom is a profuse watery diarrhea. However, the infection is treatable with Bactrim.
Malaria is a mosquito-borne (usually from female Anopheles) infectious disease caused by apicomplexans in the genus Plasmodium that affects humans and other animals. Typical symptoms include fever, malaise, vomiting, and headaches. In severe cases it can cause jaundiced skin, seizures, coma, or death. Most deaths are caused by P. falciparum since others, P. malariae, P. ovale, and P. vivax, generally cause a milder form of the disease. Symptoms usually begin ten to fifteen days after a bite from an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria. Interestingly, in the 1920s and 1930s, Arkansas had more malaria deaths than any other state. However, the protist (Plasmodium) is no longer a threat in Arkansas, as the last malaria cases (except for rare cases in travelers or immigrants, mostly from sub-Saharan Africa and South Asia) in the state was in 1951. There are several drugs (including chloroquine and antibiotics) used in treatment against the parasitic forms in the blood (the form that causes disease), and most are used in combination with quinine.
Another pathogenic coccidian that causes the disease toxoplasmosis and affects humans worldwide is Toxoplasma gondii. It is capable of infecting nearly all mammals, and almost a third of the human global population has been exposed to and may be chronically infected with the parasite. However, in immunocompetent humans, no symptoms at all may be present, or a mild, flu-like illness can occur during the first few weeks following exposure. In infants, AIDS patients, and others who are immunocompromised, infection can lead to serious health problems and sometimes death. Wild and domestic cats are considered the final hosts of T. gondii, with all other endothermic animals, including humans, serving as intermediate hosts. There are at least four routes of infection in humans and other warm-blooded animals: 1) by consuming raw or undercooked meat containing T. gondii tissue pseudocysts with bradyzoites or by sporozoites in oocysts, 2) by ingesting contaminated water, soil, vegetables, or anything possessing oocysts shed in the fecal material (like in cat litter boxes) of an infected animal, 3) from organ transplantation or via tainted blood from a blood transfusion, and 4) by transplacental transmission from mother to fetus when the mother contracts the disease during pregnancy. Treatment with pyrimethamine (Daraprim) and the antibiotic sulfadiazine is recommended.
Another apicomplexan, Neospora caninum, is closely related to T. gondii and is nearly identical in morphology. It does not affect humans; rather, it infects several domestic animals and is a major cause of abortions and stillbirth in domestic cattle worldwide. The definitive hosts are dogs, which exhibit neuromuscular disease. Dogs become infected after ingestion of infected tissues from intermediate hosts. The infection can also be transmitted congenitally, and the parasite is readily maintained in cattle and dogs by vertical transmission. A sylvatic cycle involving white-tailed deer (Odocoileus virginianus) and coyotes (Canis latrans) has also been reported.
The study of various apicomplexans in Arkansas has become more popular in the last four decades than in all years prior combined. However, several groups are yet to be studied, such as the gregarines, as none have yet been reported from any Arkansas host. Hematozoans such as Haemogregarina and Hematozoon have been reported from erythrocytes of turtles and snakes in Arkansas, respectively. There has also been an explosion in the description of new and previously described species of coccidians from various animals surveyed in the state such as amphibians (salamanders, frogs, and toads), reptiles (turtles, lizards and snakes), and mammals (particularly bats).
For additional information:
Barta, John R. “The Dactylosomatidae.” Advances in Parasitology 30 (1991): 1–37.
Dubey, Jitender P. “The History and Life Cycle of Toxoplasma gondii.” In Toxoplasma gondii: The Model Apicomplexan-Perspectives and Methods, 2nd ed., edited by L. M. Weiss and Kami Kim. New York: Elsevier, 2013.
Duszynski, Donald W., Lee Couch, and Steve J. Upton. The Coccidia of the World Database. http://www.k-state.edu/parasitology/worldcoccidia/ (accessed February 7, 2020).
Duszynski, Donald W., and J. J. Morrow. The Biology and Identification of the Coccidia (Apicomplexa) of Turtles of the World. New York: Elsevier/Academic Press Incorporated, 2014.
Duszynski, Donald W., and Steve J. Upton. The Biology of the Coccidia (Apicomplexa) of Snakes of the World: A Scholarly Handbook for Identification and Treatment. N.p.: CreateSpace Independent Publishing Platform, 2010.
Fayer, Ronald. “Sarcocystis spp. in Human Infections.” Clinical Microbiology Reviews 17 (2004): 894‒902.
Garnham, P. C. C. Malaria Parasites and Other Haemosporidia. Oxford: Blackwell Scientific, 1966.
Hill, D. E., S. Chirukandotha, and Jitender P. Dubey. “Biology and Epidemiology of Toxoplasma gondii in Man and Animals.” Animal Health Research Reviews 6 (2005): 41‒61.
Jovani, R., L. Amo, E. Arriero, O. Krone, A. Marzal, P. Shurulinkov, G. Tomas, D. Sol, J. Hagen, P. Lopez, J. Martin, C. Navarro, and J. Torres. “Double Gametocyte Infections in Apicomplexan Parasites of Birds and Reptiles.” Parasitological Research 94 (2004): 155–157.
Lainson, Ralph, I. Landau, and J. J. Shaw. “On a New Family of Non-Pigmented Parasites in the Blood of Reptiles: Garniidae Fam. Nov., (Coccidiida: Haemosporidiidea). Some Species of the New Genus Garnia.” International Journal for Parasitology 1 (1971): 241–244.
Landau, I., J. M. Chavatte, G. Karadjian, A. Chabaud, and Ian Beveridge. “The Haemosporidian Parasites of Bats with Description of Sprattiella alecto Gen. Nov., Sp. Nov.” Parasite 19 (2012): 137–146.
Landau, I., J. M. Chavatte, W. Peters, and A. Chabaud. “The Sub-Genera of Avian Plasmodium.” Parasite 17 (2010): 3–7.
Lindsay, David S., and Jitender P. Dubey. “Toxoplasmosis in Wild and Domestic Animals.” In Toxoplasma gondii: The Model Apicomplexan-Perspectives and Methods, 2nd ed., edited by L. M. Weiss and Kami Kim. New York: Elsevier, 2014.
Lindsay, David S., Jitender P. Dubey, and Byron L. Blagburn. “Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic Animals.” Clinical Microbiology Reviews 10 (1997): 19–34.
Matsche, M. A., C. R. Adams, and V. S. Blazer. “Newly Described Coccidia Goussia bayae from White Perch Morone americana: Morphology and Phylogenetics Support Emerging Taxonomy of Goussia within Piscine Hosts.” Journal of Parasitology 105 (2019): 1–10.
McAllister, C. T., and H. W. Robison. “New Host Records of Apicomplexan Blood Parasites (Haemogregarinidae and Hepatozoidae) Infecting Two Reptiles (Testudines; Ophidia) from Arkansas.” Journal of the Arkansas Academy of Science 77 (2023): 36‒41. Online at https://scholarworks.uark.edu/jaas/vol77/iss1/6/ (accessed July 22, 2024).
McAllister, Chris T. “Pfeifferinella gugleri (Apicomplexa: Pfeifferinellidae) from Roemer’s Snail, Mesodon roemeri (Gastropoda: Polygyridae), in North Central, Texas, USA.” Comparative Parasitology 80 (2013): 120‒122.
McAllister, Chris T., and Donald W. Duszynski. “The Coccidia (Apicomplexa: Eimeriidae) of Legless Lizards (Squamata: Lacertoidea: Amphisbaenia) of the World.” Journal of Parasitology 105 (2019): 113–123.
McAllister, Christ T., John A. Hnida, and Michael R. Rodriguez. “New Host and Distributional Records for Coccidian Parasites (Apicomplexa: Eimeriidae) of Vertabrates (Reptilia: Testudines, Ophidia; Aves: Passeriformes).” Comparative Parasitology 91.2 (2024): 127–131. https://doi.org/10.1654/1525-2647-91.2.127 (accessed December 26, 2024).
McAllister, Chris T., and Steve J. Upton. “The Coccidia (Apicomplexa: Eimeriidae) of Crocodylia, with Descriptions of Two New Species from Alligator mississippiensis (Reptilia: Alligatoridae) from Texas.” Journal of Parasitology 76 (1990): 332–336.
Manwell, R. D. “The Genus Dactylosoma.” Journal of Protozoology 11 (1964): 526–530.
Morrison, D. A. “Evolution of the Apicomplexa: Where Are We Now?” Trends in Parasitology 25 (2009): 375–382.
Ortega, Ynes R., Charles R. Sterling, Robert H. Gilman, Vitaliano A. Cama, and Fernando Diaz. “Cyclospora Species—A New Protozoan Pathogen of Humans.” New England Journal of Medicine 328 (1993): 1308–1312.
Pellérdy, L. P. Coccidia and Coccidiosis, 2nd ed. Berlin and Hamburg: Verlag Paul Parey, 1974.
Ramirez, N. E., L. A. Ward, and S. Sreevatsan. “A Review of the Biology and Epidemiology of Cryptosporidiosis in Humans and Animals.” Microbes and Infection 6 (2004): 773‒785.
Roberts, Larry S., and John Janovy Jr. Foundations of Parasitology. 9th ed. Boston: McGraw-Hill Higher Education, 2012.
Sam-Yellowe, T. Y. “Rhoptry Organelles of the Apicomplexa: Their Role in Host Cell Invasion and Intracellular Survival.” Parasitology Today 12 (1996): 308‒315.
Shields, J. M., and B. H. Olson. “Cyclospora cayetanensis: A Review of an Emerging Parasitic Coccidian.” International Journal for Parasitology 33 (2003): 371–391.
Smallridge, C. J., and C. M. Bull. “Transmission of the Blood Parasite Hemolivia mariae Between its Lizard and Tick Hosts.” Parasitology Research 85 (1999): 858–863.
Smith, Todd G. “The Genus Hepatozoon (Apicomplexa: Adeleina).” Journal of Parasitology 82 (1996): 565–585.
Smith, Todd G., and Sherwin S. Desser. “Phylogenetic Analysis of the Genus Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina).” Systematic Parasitology 36 (1997): 213–221.
Smith, Todd G., Sherwin S. Desser, and D. S. Martin. “The Development of Hepatozoon sipedon n. sp. (Apicomplexa: Adeleina: Hepatozoidae) in its Natural Host, the Northern Water Snake (Nerodia sipedon sipedon), the Culicine Vectors, Culex pipiens and Culex territans, and an Intermediate Host, the Northern Leopard Frog (Rana pipiens).” Parasitology Research 80 (1994): 559–568.
Telford, Sam R., Jr. Hemoparasites of the Reptilia: Color Atlas and Text. Boca Raton, FL: CRC Press, 2009.
Thompson, R. C. Andrew, Anthony Armson, and Una M. Ryan, eds. Cryptosporidium: From Molecules to Disease. Amsterdam: Elsevier, 2004.
Vannier, E., and P. J. Krause. “Human Babesiosis.” New England Journal of Medicine 366 (2012): 2397‒2407.
Wacha, R. S. “On the Taxonomic Status of the Family Pfeifferinellidae (Coccidia), with a Description of Pfeifferinella gugleri sp. n.” Journal of Protozoology 27 (1980): 368‒371.
Chris T. McAllister
Eastern Oklahoma State College
 Health and Medicine
Health and Medicine Science and Technology
Science and Technology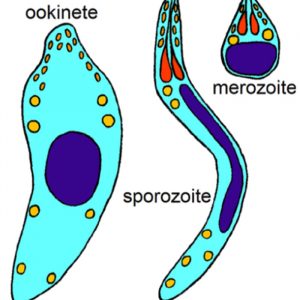 Apicomplexan Cell Types
Apicomplexan Cell Types 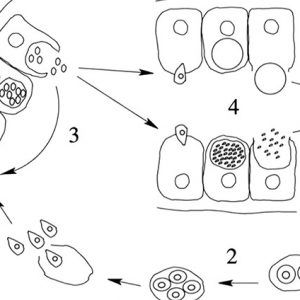 Apicomplexan Life Cycle
Apicomplexan Life Cycle 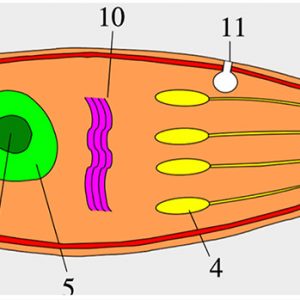 Apicomplexan Morphology
Apicomplexan Morphology 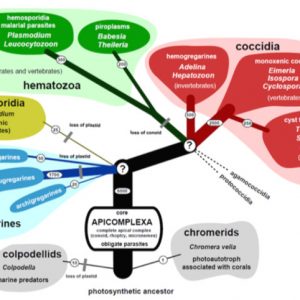 Apicomplexan Species
Apicomplexan Species 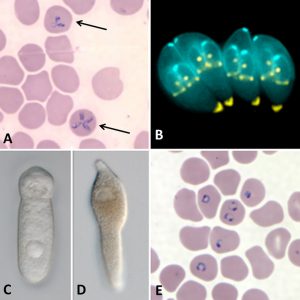 Apicomplexans
Apicomplexans 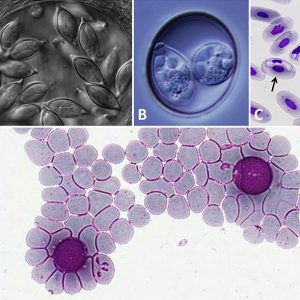 Apicomplexans Examples
Apicomplexans Examples 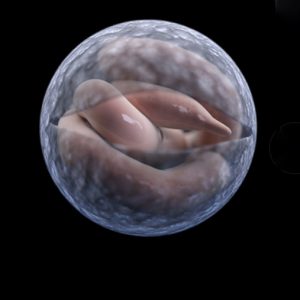 Cryptosporidium
Cryptosporidium 



Comments
No comments on this entry yet.