calsfoundation@cals.org
Shrimps
aka: Prawns
Arkansas shrimps belong in the cosmopolitan Family Palaemonidae (subfamily Palaemoninae) and Order Decapoda, which also contains the crayfishes. Taxonomically, shrimps are differentiated from crayfishes by their first pair of legs with chelae and the abdomen laterally compressed, while crayfishes have the first three pairs of legs with chelae and their abdomens dorsoventrally flattened. The Palaemonidae is a large family (950 species within 137 genera) and is distributed on all the continents except in the deep oceans, in temperate and tropical regions, and having representatives in marine, brackish, and fresh water. Representatives of this family are mainly carnivores that feed on small invertebrates. The most prominent genus in the family is Macrobrachium, with over 240 species that include commercially fished species; a single representative occurs in Arkansas, the Ohio Shrimp, M. ohione. The Ohio shrimp is the only endemic species of the genus in North America, and it is the most widely distributed and abundant river shrimp in the United States. The other shrimp representative in Arkansas is the glass (or Mississippi grass) shrimp, Palaemonetes kadiakensis.
All six species of Macrobrachium in the United States are amphidromous—that is, they spawn in salt water and need to migrate upstream to complete their life cycles. Therefore, M. ohione has an amphidromous life history in which spawning is not dependent on salt or freshwater, but early development requires salt or brackish water. It is thought that the genus Macrobrachium only recently evolved migratory behavior into fresh water. Biological characteristics indicative of this relatively recent adaptation include high hemolymph osmo-ionic concentrations, tolerance of high salinities, dependence on saline waters for larval development with many larval stages, and migratory behavior.
The Ohio shrimp is the larger (up to 110 mm or 4.3 in., average length 60 mm or 2.4 in. long) of the two species inhabiting Arkansas. In one study conducted in the state, the largest Ohio shrimp collected was a female that was 68 mm (2.7 in.) in total length. Females are typically larger than males, although ovigerous females as small as 35 mm (1.4 in.) in total length are known.
Historically, until about the mid-1930s, M. ohione were consumed extensively and sold as bait for fisheries in the Mississippi and Ohio river systems. Commercial fisheries still exist in Louisiana, Mississippi, and Texas predominately for the purpose of fish bait, especially for use by commercial fishermen catching catfishes on trotlines.
The Ohio shrimp occurs in the Mississippi River basin, in Gulf Coastal drainages, and also in some Atlantic coast drainages from Florida and Georgia north to Virginia. It has been collected in several states along the Gulf Coastal Plain and Atlantic Coastal Plain states, including Alabama, Florida, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, Texas, and Virginia. This species also occurs in the coastal streams of northeastern Mexico. In the Mississippi River Basin proper, specimens have been collected in Arkansas, Missouri, Illinois, Indiana, Ohio, and Oklahoma. In Arkansas, historical collections have been made in the Arkansas River as far upriver as Fort Smith (Sebastian County).
Prior to 2018, collections of M. ohione made in Chicot and Phillips counties, along the Mississippi River, revealed a total of forty-two specimens collected and vouchered in Arkansas in the last 100 years. These specimens were collected either by seining or dip netting. However, no ovigerous females (those carrying eggs among the pleopods) had previously been collected in the state. A more recent unpublished study conducted along the mainstem Mississippi River and lower Arkansas, White, and St. Francis rivers using wire mesh traps (galvanized hardware cloth) reported a total of 6,984 Ohio shrimp taken, with 5,090 (73%) of those individuals classified as juveniles. In addition, in the same study, collections were made of twenty-two ovigerous females, marking the first study that has observed females with eggs that far upriver from their estuarine environment (more than 800 river km [497 mi.]).
Macrobrachium ohione predominately scavenges on dead plant and animal material while sifting substrate. It has also been observed to be omnivorous, capable of catching small fish and consuming larger fish in traps. In terms of predators, M. ohione has been documented to be eaten by a variety of freshwater fish that occur in Arkansas, including Aplodinotus grunniens, Ictalurus furcatus, Micropterus salmoides, Morone mississippiensis, Pylodictis olivaris, and Scaphirhynchus platorynchus.
Regarding reproduction in M. ohione, maximum migration for upriver movement has been documented in July, and the reproductive period begins in March and ends by September. External fertilization in fresh water as well as salt water occurs in M. ohione whereby eggs are fertilized as they are excreted from the gonopore of the female. Females carry their fertilized eggs for fifteen to twenty days within the pleopods until they develop enough to hatch. After hatching, young individuals undergo a series of developmental stages called zoea. The transition from zoea stage-1 (first larval instar) to stage-2 zoea requires salt water to develop. Stage-1 larvae do not feed and rely on yolk droplets left over from the embryo, while stage-2 larvae begin to feed and go into further larval stages. Once shrimp grow to the final zoea stage, which could require as many as ten instars, they metamorphose into the post-larval stage when moving upriver. The total time period from hatching to a juvenile size of 25 mm (1.0 in.) in total length is about 71 to 129 days.
The Ohio shrimp is characterized by having the first pairs of legs chelate, the second pair larger than the first, the carpus of the second leg not subdivided, a hepatic spine present, the upper edge of the rostrum curved with nine to thirteen teeth and a toothless dagger-like tip, and the second pereopods enlarged and greatly elongated. The Ohio shrimp is the least colorful of the other five species (M. acanthurus, M. carcinus, M. faustinum, M. heterochiris, and M. olfersii) occurring in the United States. The base color is pale gray to olivaceous with light blue spots and a blue telson and uropod.
The Ohio shrimp inhabits open side channels and main channel borders of the Mississippi River, as well as low-velocity waters. These shrimp tend to prefer the borders of the river channels, especially when the borders are flooded and organic material is available for foraging. Most Arkansas specimens have been seined over sandy substrates in 61 to 91 cm (2 to 3 ft.) of water without vegetation and no appreciable current offshore adjacent to sandbars. This shrimp is thought to receive reproductive cues from spring floods and use flooded terrestrial habitat for reproduction.
The Ohio shrimp has declined in abundance drastically since the 1930s in northern areas of its former range. It was formerly abundant in the Mississippi River as far north as Chester, Illinois (and possibly St. Louis, Missouri), and in the Ohio River as far upstream as southeastern Ohio. Reasons for its decline include overharvesting, river channelization, dredging, levee and dam construction, water pollution, and habitat loss. This shrimp must have a direct and/or unobstructed connection with estuarine areas to ensure reproduction. In Arkansas, the overall status of upriver populations of M. ohione should be of conservation concern. It had originally been considered a “threatened” species in Arkansas because of this restricted distributional range. However, M. ohione appears to have a stable population in its overall downriver range and has been listed on the IUCN Red List of Threatened Species as a “species of least concern.”
Palaemonetes kadiakensis is one of thirty members of the genus Palaemonetes. It ranges from northeastern Mexico, north to the Great Lakes, and east to Florida. Compared to M. ohione, the smaller glass shrimp reaches a total length of up to 53 mm (2.1 in.). In the past, this smaller shrimp has been used as fish bait as well as for fish forage in farm ponds.
Glass shrimp are differentiated from M. ohione by having the second pair of legs only slightly longer than the first pair, only six to eight teeth occurring along the upper edge of the rostrum, and a branchiostegal spine, but no hepatic spine is present. Living specimens are transparent, with green eyes, red-brown antennae, and many very small red-brown specks on the body. Often, a bright green vegetation-filled intestine is apparent.
The glass shrimp inhabits vegetated areas of lentic and slower-moving streams as well as the sheltered areas of more rapidly flowing environs below the Fall Line in Arkansas. It is also commonly found in the vegetated backwaters of the Mississippi River and in swamps and low-gradient streams of eastern Arkansas. When it inhabits large rivers, P. kadiakensis is generally found associated with low-velocity waters and vegetation. In adjacent Oklahoma, this shrimp has been found in large rivers, large turbid impoundments, and small turbid ponds filled with aquatic vegetation in the flood plains of large rivers or new pools and bar ditches filled by recent floodwaters. It does best in pools with low fish abundance. These shrimp seem to like high visibility and low water velocity.
In Arkansas, glass shrimp are commonly found in sluggish backwater regions of Coastal Plain streams, especially preferring heavily vegetated lentic areas of pool regions. For example, Big Creek (Bayou Dorcheat drainage) in Columbia County, where P. kadiakensis is quite common, is typical of Arkansas waters inhabited by this shrimp. While the water is normally non-turbid, Big Creek has an obvious darkly stained, reddish-brown hue imparted to the water by tannins leached from an abundance of organic matter in the stream. Glass shrimp also seem to have an association with submerged and semi-aquatic vegetation (Myriophyllum spp., Polygonum hydropiperoides var. opelosanum, Proserpinaca palustris) which is almost always abundant. Seining of thick vegetation near the edge of the main channel of lowland streams where current is reduced or absent often yields specimens.
Typically, P. kadiakensis inhabits areas below the Fall Line zone in the Gulf Coastal Plain physiographic province of Arkansas, but it is known to occur westward in the Arkansas Valley continuing west into Oklahoma. In Arkansas, most counties that support populations of P. kadiakensis lie within the Gulf Coastal Plain physiographic province. A single collection in the U.S. National Museum is known from North Sylamore Creek near Fifty-Six (Stone County). This represents the farthest known penetration of P. kadiakensis into the Ozark Mountains within Arkansas.
Over its range in the United States, this shrimp reproduces from May through August with a peak in mid-June. In Illinois, reproduction occurs from April to August, while in Louisiana, the reproductive period extends from February to October and in Missouri from May to August. In Arkansas, studies indicate that reproduction occurs from April to July. The post-reproductive individuals die, and the larvae grow rapidly, obtaining fifty percent of their ultimate length in the first three months of life. Arkansas populations seem to be made up of individuals that hatch in the summer, grow during the autumn months, survive the winter, and then reproduce and die in the late spring to early fall, thus having only a one-year life cycle.
Glass shrimp are reported to reach a total length of 53 mm (2.1 in.) in Louisiana, while a 46 mm (1.8 in.) specimen has been reported from Oklahoma. The smallest Arkansas specimen is 13 mm (0.5 in.) in total length while the largest is 32 mm (1.3 in.). Females are generally slightly larger than males, while gravid females are almost always much larger. Sex ratio of the glass shrimp collected in Arkansas is 1:1.9. Ovigerous females have been collected in Arkansas in April, May, June, and July. Thirteen selected females collected in Arkansas yielded 38 to 141 (mean 67.7) eggs (embryos).
With regard to conservation status of the glass shrimp in Arkansas, this shrimp has remained relatively abundant and widespread in occurrence since the late twentieth century in the state, has a stable conservation status, and is in no need of special protection. The Nature Conservancy lists populations of P. kadiakensis as secure (G5) in rounded global status.
In the early twenty-first century, there was a short-lived interest by a small aquaculture operation in Wilmot (Ashley County) in developing freshwater prawns (Macrobrachium rosenbergii) and Pacific white shrimp or king prawn (Litopenaeus vannamei) as aquaculture alternatives in Arkansas. The small local operation was started in the hope of becoming a national leader in domestic shrimp production, but it was apparently unsuccessful. In general, M. rosenbergii production is an expensive business, and potential producers must carefully take into account the costs and risks involved. In addition, L. vannamei is a tropical species that cannot survive winters in Arkansas. Personnel at the Aquaculture/Fisheries Center at the University of Arkansas at Pine Bluff have been interested in raising shrimp in the state.
Concerning parasites of these two shrimps, Ohio shrimp from Arkansas have been found to be infested with the bopyrid isopod, Probopyrus pandalicola. Parasites occur in the space between the inner carapace wall and gills of the host. Infection of adult M. ohione is common in the Atchafalaya and Mississippi rivers in Louisiana. Parasites have not yet been reported from P. kadiakensis from Arkansas, but specimens from other states have yielded various species as follows: a digenetic trematode, Alloglossidium renale, has been reported from the antennary gland of P. kadiakensis from the Mississippi River of Louisiana and Alabama; a ciliophoran (Lagenophrys verecunda) was described from the gill lamellae of P. kadiakensis from Florida; and aquatic fungi (Saprolegnia parasitica and Achlya flagellata) infected laboratory-reared larval P. kadiakensis.
For additional information:
Anderson, G. “Observations on the Distribution and Movements of Macrobrachium ohione (Smith, 1874) in the Pascagoula River Estuary, Jackson County, Mississippi, U.S.A. (Decapoda, Palaemonidae).” Crustaceana 44 (1983): 123–140.
Barko, Valerie A., and David P. Herzog. “Relationships among Side Channels, Fish Assemblages, and Environmental Gradients in the Unimpounded Upper Mississippi River.” Journal of Freshwater Ecology 18 (2003): 377–382.
Barko, Valerie A., and Robert A. Hrabik. “Abundance of Ohio Shrimp (Macrobranchium ohione) and Glass Shrimp (Palaemonetes kadiakensis) in the Unimpounded Upper Mississippi River.” American Midland Naturalist 151 (2004): 265–273.
Bauer, Raymond T. Remarkable Shrimps: Adaptations and Natural History of the Carideans. Norman: University of Oklahoma Press, 2004.
Bauer, Raymond T., and James Delahoussaye. “Life History Migrations of the Amphidromous River Shrimp, Macrobranchium ohione From a Continental Large River System.” Journal of Crustacean Biology 28 (2008): 622–632.
Bouchard, Raymond W., and Henry W. Robison. “An Inventory of the Decapod Crustaceans, Crayfishes and Shrimps, of Arkansas.” Proceedings of the Arkansas Academy of Science 34 (1980): 22–30. Online at: https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2643&context=jaas (accessed December 6, 2019).
Bowles, David E., Karim Aziz, and Charles L. Knight. “Macrobranchium (Decapoda: Caridea: Palaemonidae) in the Contiguous United States: A Review of the Species and An Assessment of Threats to Their Survival.” Journal of Crustacean Biology 20: 158–171.
Carney, J. P., and Daniel R. Brooks. “Phylogenetic Analysis of Alloglossidium Simer, 1929 (Digenea: Plagiorchiiformes: Macroderoididae) with Discussion of the Origin of Truncated Life Cycle Patterns in the Genus.” Journal of Parasitology 77 (1991): 890–900.
Cheper, Nicholas J. “Palaemonetes kadiakensis Rathbun in Oklahoma (Crustacea: Decapoda).” Proceedings of the Oklahoma Academy of Science 68 (1988):77–78.
———. “Palaemonetes kadiakensis (Crustacea: Decapoda) in Oklahoma, 1982 and 1987.” Proceedings of the Oklahoma Academy of Science 72 (1992): 65.
Conaway, L. K. and Robert A. Hrabik. “The Ohio Shrimp, Macrobranchium ohione, in the Upper Mississippi River.” Transactions of the Missouri Academy of Science 31 (1997): 44–46.
Cooper, John E. “Giant River Shrimps of the Genus Macrobrachium (Decapoda: Palaemonidae) in North and South Carolina.” Journal of the North Carolina Academy of Science 127 (2011): 176–178.
Hedgpeth, J. W. “The North American Species of Macrobranchium (River Shrimp).” Texas Journal of Science 1 (1949): 28–38.
Hubscham, Jerry, and Jo Ann Rose. “Palaemonetes kadiakensis Rathbun: Post Embryonic Growth in the Laboratory (Decapoda, Palaemonidae).” Crustaceana 16 (1969): 81–87.
Landers, S. C., and R. D. Jones. “Pathology of the Trematode Alloglossidium renale in the Freshwater Grass Shrimp Palaemonetes kadiakensis.” Southeastern Naturalist 8 (2009): 599–608.
Nielsen, L. A., and J. S. Reynolds. “Population Characteristics of a Freshwater Shrimp Palaemonetes kadiakensis Rathbun.” Transactions of the Missouri Academy of Science 10/11 (1975): 44–57.
Olivier, Tyler J. “Amphidromous Life History of the Caridean Shrimp Macrobrachium ohione (Decapoda: Palaemonidae) from the Mississippi River System.” PhD diss., University of Louisiana at Lafayette. ProQuest/UMI (Publication No. 3590041), 2013.
Olivier, Tyler J., and Raymond T. Bauer. “Female Downstream-Hatching Migration of the River Shrimp Macrobrachium ohione in the Lower Mississippi River and the Atchafalaya River.” American Midland Naturalist 166 (2011): 379–393.
Olivier, Tyler J., Sara L. Conner, and Raymond T. Bauer. “Evidence of Extended Marine Planktonic Larval Development in Far-Upstream Populations of the River Shrimp Macrobrachium ohione (Smith, 1874) from the Mississippi River.” Journal of Crustacean Biology 32 (2012): 899–905.
Pigg, Jimmie, and Nicholas J. Cheper. “Additional Observations on the Distribution and Habitats of Palaemonetes kadiakensis Rathbun (Crustacea: Decapoda) in Oklahoma, 1992 to 1996.” Proceedings of the Oklahoma Academy of Science 78 (1998): 119–122.
Poly, William J., and James E. Wetzel. “The Ohio Shrimp, Macrobranchium ohione (Palaemonidae), in the Lower Ohio River.” Transactions of the Illinois Academy of Science 95 (2002): 65–66.
Reimer, Robert D., K. Strawn, and A. Dixon. “Notes on the River Shrimp, Macrobrachium ohione (Smith) 1874, in the Galveston Bay System of Texas.” Transactions of the American Fisheries Society 103 (1974):120–126.
Robison, Henry W., and Chris T. McAllister. “Geographic Distribution and Life History Aspects of the Freshwater Shrimps, Macrobrachium ohione and Palaemonetes kadiakensis (Decapoda: Palaemonidae), in Arkansas.” Journal of the Arkansas Academy of Science 65 (2011): 98–110. Online at: https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1347&context=jaas (accessed December 6, 2019).
Robison, Henry W., Lindsay Lewis, C. Cox, Geoffry Spooner, Reid Adams, and Chris T. McAllister. “New Distributional Records of the Ohio Shrimp, Macrobrachium ohione Smith (Decapoda: Palaemonidae).” Journal of the Arkansas Academy of Science 70 (2016): 207–210. Online at: https://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2204&context=jaas (accessed December 6, 2019).
Spooner, Geoffry L. “The Migration Dynamics of the Ohio Shrimp, Macrobranchium ohione, in the Lower Mississippi River.” Master’s thesis, University of Central Arkansas, Conway, 2018.
Stone, Nathan, George Selden, and Anita M. Kelley. Aquaculture Alternatives in Arkansas. Cooperative Extension Program, University of Arkansas at Pine Bluff. Publication FSA9055-PD-11-12RV. Online at https://www.uaex.edu/publications/PDF/FSA-9055.pdf (accessed December 6, 2019).
Taylor, Christopher A. “The Rediscovery of the Ohio Shrimp, Macrobrachium ohione, in Illinois.” Transactions of the Illinois State Academy of Science 85 (1992): 227–228.
Thoma, Robert F., and R. E. Jezerinac. “Ohio Crayfish and Shrimp Atlas.” Ohio Biological Survey Miscellaneous Contribution 7 (2000): 1–28.
Henry W. Robison
Sherwood, Arkansas
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology Palaemonetes kadiakensis
Palaemonetes kadiakensis 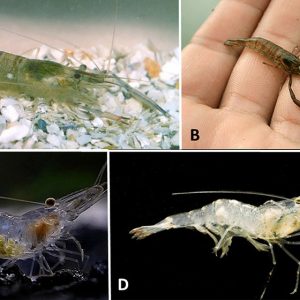 Shrimps of Arkansas
Shrimps of Arkansas 



Comments
No comments on this entry yet.