calsfoundation@cals.org
Mantodea
aka: Mantids
aka: Mantises
Mantises belong to the Phylum Arthropoda, Class Insecta, and Order Mantodea. The order contains over 2,400 species within about 430 genera in fifteen families. The largest family is the cosmopolitan Mantidae. Mantises are distributed worldwide in mostly tropical but also temperate habitats. Little has been published on the mantises of Arkansas, although the Carolina mantid, Stagmomantis carolina, has been investigated in the central part of the state.
In the fossil history of mantises, fossils of the group are rare. For example, by 2007, only about twenty-five fossil species were known. The earliest, from Siberia, are about 135 million years old. Fossil mantises, from Cretaceous amber, include a specimen from Japan with spines on the front legs, as in modern mantises. Most fossils in amber are nymphs, whereas compression fossils (in rock) include adults. Fossil mantises from 110 million years ago from the Lower Cretaceous Crato Formation in Brazil include the 10 mm (0.39 in.) long Santanmantis axelrodi, described in 2003. As in modern varieties, its front legs were adapted for catching prey.
The systematics of mantises have long been debated. Along with stick insects (Phasmatodea), mantises were once placed in the order Orthoptera with the cockroaches (now the Blattodea) and rock crawlers (now Grylloblattodea). In one study published in 1991, it was suggested to combine the Mantodea with the cockroaches and termites into the order Dictyoptera, suborder Mantodea. The closest relatives of mantises are the termites and cockroaches, which are all within the superorder Dictyoptera.
One of the earliest classifications splitting an all-inclusive Mantidae into multiple families was proposed in 1968 that recognized eight families. Later, in 2002, reclassification of the order increased the number to fifteen families. By 2020, about fifteen families were recognized, including two that are extinct. In 1997, in a study of the external male genitalia, it was postulated that the Neotropical family Chaeteessidae and Southeast Asian family Metallyticidae diverged from the other families at an early date. However, the Mantidae and Neotropical Thespidae from India and Indo-China through to Papua New Guinea are still both considered polyphyletic, so the Mantodea will eventually have to be revised.
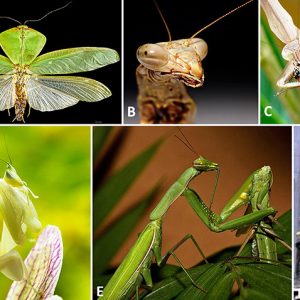
Morphologically, mantises have large, triangular heads with bulging eyes supported on flexible necks and a beak-like snout and mandibles. They have a pair of antennae, two bulbous compound eyes, and three small simple eyes. The articulation of the neck is also a remarkable adaptation; it is very flexible and, in some species, it can be rotated on the head nearly 180 degrees. The thorax consists of a prothorax, a mesothorax, and a metathorax. In all species, except eleven species in the genus Mantoida native to Mexico and Central and South America, the prothorax, which conveys the head and forelegs, is much longer than the other two thoracic segments. The prothorax is also flexibly articulated, allowing for a wide range of movements of the head and forelimbs while the remainder of the body remains more or less immobile.
All Mantodea have forelegs that are greatly enlarged and tailored for catching and gripping prey; their upright posture, while remaining stationary with forearms folded, has led to their common name, praying mantis. Their two spiked, raptorial legs (grasping forelegs) are used to capture prey items and hold them securely. In these raptorial legs, the coxa and trochanter combine to form a segment about the length of the femur, which is a thorny part of the grasping apparatus. Positioned at the base of the femur is a set of discoidal spines, usually four in number. These spines are preceded by a number of tooth-like tubercles, which, along with a similar series of tubercles along the tibia and the apical claw near its tip, give the foreleg of the mantis its hold on its prey. The foreleg ends in a subtle tarsus used as a walking appendage, made of four or five segments and ending in a two-toed claw with no arolium.
Their elongated bodies may or may not have wings. Therefore, mantises can be loosely sorted as being long-winged (macropterous), short-winged (brachypterous), vestigial-winged (micropterous), or wingless (apterous). If not the latter category, a mantis has two sets of wings. The outer wings (tegmina) are usually narrow and leathery. They function as a shield and camouflage for their hind wings, which are clearer and more delicate. In all mantises, the abdomen consists of ten tergites, with a corresponding set of nine sternites visible in males and seven visible in females. The abdomen ends in a pair of cerci in both sexes but tends to be slimmer in males versus females.
In terms of vision in insects, mantises are a very advanced group that possesses stereovision in which they locate their prey by sight; their compound eyes contain up to 10,000 ommatidia. A small region at the anterior called the fovea has greater visual acuity than the rest of the eye and can produce the high resolution necessary to examine potential prey. The peripheral ommatidia are concerned with detecting motion; when a moving object is noticed, the head is rapidly rotated to transmit the object into the visual field of the fovea. Further prey motions are then tracked by movements of the mantis’s head so as to keep the image centered on the fovea. Because the eyes are laterally situated and widely spaced, it affords a wide binocular field of vision and, at close range, precise stereoscopic vision. The dark spot on each eye is a pseudopupil that moves as the insect rotates its head. This occurs because the ommatidia that are viewed “head-on” absorb the incident light, while those to the side redirect it.
Since their hunting ability relies profoundly on vision, mantises are primarily daytime, diurnal species. Many species, however, fly at night, and may be attracted to artificial lights. Nocturnal mantises in the Neotropical family Liturgusidae, found from India to Australia, have been shown to be predominately male, which is probably true for most mantises. Nocturnal flight is especially important to males in detecting pheromones of less-mobile females. Also, flying at night exposes mantises to fewer bird predators than diurnal flight would. Many mantises also have an auditory thoracic organ that helps them avoid bats (Chiroptera) by detecting their echolocation calls and responding evasively. When frequencies begin to increase rapidly, indicating an approaching bat, mantises stop flying horizontally and begin a descending spiral toward the safety of the ground, often preceded by an aerial loop or spin. If caught, they may slash captors with their raptorial legs.
Mantises are mostly ambush predators of arthropods that only feed upon live prey within their reach. Once within reach, mantises assault rapidly with their spiked raptorial forelegs to grasp their prey. However, a few species that dwell on the ground or on bark are found actively pursuing their prey. They either camouflage themselves and become “sit and wait” predators or stalk tempting prey with slow, stealthy movements. For example, members of three genera, such as the ground mantises Entella, Ligaria, and Ligariella, travel overland seeking prey. Larger mantises sometimes cannibalize smaller individuals of their own species, as well as seek small vertebrates such as fishes, frogs, lizards, and small birds.
Predators of mantises include vertebrates such as frogs, lizards, and birds, and invertebrates such as spiders, large hornets, and ants. Some hunting wasps, such as some species of Tachytes, also feed their young by paralyzing some species of mantis. Ants that are predators of mantises include the genera Loxomantis, Orthodera, and Statilia. To protect themselves, mantises use camouflage. In fact, most species are cryptically colored to resemble foliage or other backgrounds, both to avoid predators and to snare their prey better. Those that live on uniformly colored surfaces such as bare earth or tree bark are dorsoventrally flattened so as to eliminate shadows that might reveal their presence. The species from different families called flower mantises are aggressive mimics: they resemble flowers convincingly enough to attract prey that come to collect pollen and nectar. For example, Malaysian orchid mantises (Hymenopodidae) of Southeast Asia might be the greatest tricksters of the animal kingdom; they are camouflaged pink or yellow, matching the coloration of local orchids. This mantis mimics live orchids so well that it dupes pollinating bugs that stop by for a visit; instead of collecting pollen and nectar, the bugs become a meal for the mantis. In addition, some species in Africa and Australia are able to turn black after a molt toward the end of the dry season; at this time of year, bush fires occur and this coloration enables them to blend in with the fire-ravaged landscape (a form of fire melanism).
When directly threatened, many mantis species stand tall and spread their forelegs, with their wings fanning out wide. The fanning of the wings makes the mantis appear larger and more threatening, with some species enhancing this effect with bright colors and patterns on their hind wings and inner surfaces of their front legs. If the harassment continues, a mantis may strike out with its forelegs and attempt to pinch or bite the intruder. As part of the bluffing (deimatic) threat display, some species may also produce a hissing sound by expelling air from the abdominal spiracles.
Similar to stick insects, mantids exhibit a rocking behavior in which the individual makes rhythmic, cyclic side-to-side movements. The function for this behavior is thought to include the enhancement of crypsis by means of the resemblance to vegetation moving in the wind. However, the repetitive swaying movements may be more important in allowing the insects to discriminate objects from the background by their relative movement, a visual mechanism typical of animals with simpler vision systems. Rocking movements by these generally sedentary insects may also replace flying or running as a source of relative motion of objects in the visual field. Taking advantage of this behavior, a variety of arthropods, including some early-instar mantises, mimic ants to evade predation.
In temperate climates, the reproductive season of mantises typically takes place in the fall (followed by the female dying after oviposition), while in tropical areas, mating can occur year round. Following courtship, the male usually leaps onto the female’s back and clasps her thorax and wing bases with his forelegs. For successful copulation, he then arches his abdomen to deposit and store sperm in a special chamber near the tip of the female’s abdomen. Interestingly, females sometimes practice sexual cannibalism, eating their mates after copulation. Depending on the species, the female lays between ten and 400 eggs. They are typically deposited in a froth mass-produced by glands in the abdomen. This lather solidifies, creating a protective capsule, which together with the egg mass is called an ootheca. Depending on the species, the ootheca can be attached to a flat surface, wrapped around a plant, or even deposited in the ground. Despite the versatility and resilience of the eggs, they are often preyed on, especially by several species of parasitoid wasps. In a few species, mostly ground and bark mantises (family Tarachodidae) of Africa, Madagascar, Indian Ocean Islands, and India, the mother guards the eggs. The cryptic Tarachodes maurus positions herself on bark with her abdomen covering her egg capsule, ambushing passing prey and remaining mostly stationary until the eggs hatch. An unusual reproductive strategy is also espoused by Brunner’s stick mantis (Brunneria borealis) from the southern United States; no males have ever been found in this species, so the females breed parthenogenetically. The ability to reproduce by parthenogenesis has also been recorded in at least two other species, the African mantis (Sphodromantis viridis) and a Miomantis sp., although these species usually reproduce sexually. In temperate climates, adults do not survive the winter, and the eggs undergo a diapause, hatching in the spring.
As in closely related insect groups in the superorder Dictyoptera, mantises go through three life stages: egg, nymph, and adult (mantises are among the hemimetabolous insects). For smaller species, the eggs may hatch in three to four weeks as opposed to larger species, which take four to six weeks. The nymphs may be colored differently from the adult, and the early stages are often mimics of ants. A mantis nymph grows bigger as it molts its exoskeleton. Depending on the species, molting can happen five to ten times before adulthood is reached. After the final molt, most species have wings, though some species remain wingless or short-winged, particularly in females. The lifespan of a mantis depends on the species; smaller ones may live four to eight weeks, while larger species may live four to six months.
Sexual cannibalism is common among most predatory species of mantises in captivity. Around 90 percent of the predatory species of mantises exhibit sexual cannibalism. It has sometimes been observed in natural populations, where about a quarter of male-female encounters result in the male being eaten by the female. At first, adult males typically outnumber females, but their numbers may be fairly equivalent later in the adult stage, possibly because females selectively eat the smaller males. Eighty-three percent of Chinese mantis (Tenodera sinensis) males escape cannibalism after an encounter with a female, but since multiple matings occur, the probability of a male being eaten increases cumulatively. The female may begin feeding by chomping off the male’s head (as they do with regular prey), and if mating has begun, the male’s movements may become even more vigorous in its delivery of sperm.
Mantises are among the insects most commonly kept as pets. Because the lifespan of a mantis in captivity is, at best, about a year, people who keep mantises often breed them. In 1996, at least fifty species were known to be kept in captivity by members of the Mantis Study Group. In 2013, at least thirty-one species were kept and bred in the United Kingdom, the Netherlands, and the United States.
For pest control, gardeners who prefer to avoid using pesticides may encourage mantises in the hope of controlling insect pests. However, mantises have negligible value in pest control and do not have the key attributes of biological pest control agents; they do not specialize in a single pest insect, and do not multiply rapidly in response to an increase in such a prey species, but are general predators. They eat whatever they can catch, including both harmful and beneficial insects.
Two species, T. sinensis (from China in 1896) and the European mantis, Mantis religiosa (from Europe in 1899) were deliberately introduced into North America in the hope that they would serve as pest controls for agriculture; however, they have spread widely in both the United States and Canada. Other non-native (exotic) species in North America include Liturgusa maya (Liturgusidae) recently from the Neotropics; narrow-winged mantis, Tenodera angustipennis (Mantidae), from Asia in 1930; and the Mediterranean mantis, Iris oratoria (Tarachodidae), from the Old World.
Not much has been published specifically on the mantises of Arkansas. However, the life history and population characteristics of the Carolina mantid, Stagmomantis carolina, have been investigated in the central part of the state.
For additional information:
Arnett, Ross H. American Insects: A Handbook of the Insects of America North of Mexico. Boca Raton, FL: CRC Press, 2000.
Beckman, Noelle, and Lawrence E. Hurd. “Pollen Feeding and Fitness in Praying Mantids: The Vegetarian Side of a Tritrophic Predator.” Environmental Entomology 32 (2003): 881–885.
Bragg, P. E. “A Case of Parthenogenesis in a Mantis.” Bulletin of the Amateur Entomologists’ Society 46 (1987): 160.
Gelperin, Alan. “Feeding Behaviour of the Praying Mantis: A Learned Modification.” Nature 219 (1968): 399–400.
Harris, S. J., and M. D. Moran. “Life History and Population Characteristics of the Mantid Stagmomantis carolina (Mantodea: Mantidae).” Environmental Entomology 29 (2000): 64‒68.
Hurd, L. E., R. M. Eisenberg, W. F. Fagan, K. J. Tilmon, W. E. Snyder, K. S. Vandersall, S. G. Datz, and J. D. Welch. “Cannibalism Reverses Male-Biased Sex Ratio in Adult Mantids: Female Strategy against Food Limitation?” Oikos 69 (1994): 193–198.
Lawrence, S. E. “Sexual Cannibalism in the Praying Mantid, Mantis religiosa: A Field Study.” Animal Behaviour 43 (1992): 569–583.
Lelito, Jonathan P., and William D. Brown. “Complicity or Conflict over Sexual Cannibalism? Male Risk Taking in the Praying Mantis Tenodera aridifolia sinensis.” American Naturalist 168 (2006): 263–269.
Liske, E., and W. J. Davis. “Courtship and Mating Behaviour of the Chinese Praying Mantis, Tenodera aridifolia sinenesis.” Animal Behaviour 35 (1987): 1524–1537.
———. “Sexual Behaviour of the Chinese Praying Mantis.” Animal Behaviour 32 (1984): 916–918.
Maxwell, Michael R. “Lifetime Mating Opportunities and Male Mating Behaviour in Sexually Cannibalistic Praying Mantids.” Animal Behaviour 55 (1998): 1011–1028.
Milne, Lorus, and Margery Milne. National Audubon Society Field Guide to Insects and Spiders. New York: Albert R. Knopf, 1980.
Nelson, Ximena J., and Robert R Jackson. “Innate Aversion to Ants (Hymenoptera: Formicidae) and Ant Mimics: Experimental Findings from Mantises (Mantodea).” Biological Journal of the Linnean Society 88 (2006): 23–32.
Nyffeler, Martin, Michael R. Maxwell, and J. V. Remsen Jr. “Bird Predation by Praying Mantises: A Global Perspective.” Wilson Journal of Ornithology 129 (2017): 331–344.
O’Hanlon, James C., Gregory I. Holwell, and Marie E. Herberstein. “Pollinator Deception in the Orchid Mantis.” American Naturalist 183 (2014): 126–132.
Prete, Frederick R., and K. S. Cleal. “The Predatory Strike of Free Ranging Praying Mantises, Sphodromantis lineola (Burmeister). I: Strikes in the Mid-Sagittal Plane.” Brain, Behavior and Evolution 48 (1996): 173–190.
Prete, Frederick R., Lawrence E. Hurd, Patrick H. Wells, and Harrington Wells. The Praying Mantis. Baltimore: Johns Hopkins University Press, 2000.
Ross, Edward S. “Mantids—The Praying Predators.” National Geographic 165 (1984): 268–280.
Rossel, S. “Binocular Vision in Insects: How Mantids Solve the Correspondence Problem.” Proceedings of the National Academy of Sciences of the United States of America 93 (1996): 13229–13232.
Whitcomb, W. H., and Kermit Bell. Predaceous Insects, Spiders, and Mites of Arkansas Cotton Fields. Agricultural Experiment Station Bulletin 690. Fayetteville: University of Arkansas Agricultural Experiment Station, 1964.
Wilder, Shawn M., Ann L. Rypstra, and Mark A. Elgar. “The Importance of Ecological and Phylogenetic Conditions for the Occurrence and Frequency of Sexual Cannibalism.” Annual Review of Ecology, Evolution, and Systematics 40 (2009): 21–39.
Chris T. McAllister
Eastern Oklahoma State College






Comments
No comments on this entry yet.