calsfoundation@cals.org
Hemipterans
aka: True Bugs
Hemipterans, or true bugs, belong to the Phylum Arthropoda, Superclass Hexapoda, Class Insecta, Order Hemiptera, and four suborders: Auchenorrhyncha, Coleorrhyncha, Sternorrhyncha, and Heteroptera. Hemiptera is the largest order of hemimetabolous insects (those not undergoing complete metamorphosis), although male scale insects (Coccoidea) do undergo a form of complete metamorphosis. The number of species in the order is about 75,000, with a great diversity of forms, including aphids, cicadas, leafhoppers, planthoppers, and shield bugs. The three largest families of Heteroptera are Miridae (plant bugs), Lygaeidae (seed bugs), and Pentatomidae (stink bugs). The largest family, Miridae, contains major insect pests and predatory groups that can be used as biological control agents. Although hemipterans inhabit a wide variety of habitats, most are generally terrestrial, including a number of important agricultural pests, with some species adapted to life in fresh water or on the surface, including those with aquatic larvae or nymphs. These include the water boatmen (Corixidae), backswimmers (Notonectidae), water striders (Gerridae), waterscorpions (Nepidae), and giant water bugs (Belostomatidae). In addition, sea skaters (Gerridae) in the genus Halobates are the only insects that are truly marine; they occur on the surface of the Pacific Ocean. About forty species are found in subtropical and tropical marine habitats around the world, with one species recorded in rivers a few kilometers upstream from the ocean. In Arkansas, much of the research on Hemipterans has been done by Arkansas State University (ASU) faculty members and their students.
Historically, the present members of the order Hemiptera (also referred to as Rhynchota) were placed into two orders, the Homoptera and Heteroptera/Hemiptera, based on morphological differences in the position of their rostrum and wing structure. Now, the order has been divided into four or more suborders since the Homoptera were established as paraphyletic (now the Auchenorrhyncha and the Sternorrhyncha). This phylogenetic analysis was based on homogeneous models and DNA analyses (mitochondrial genome sequences). This study now places the Sternorrhyncha as sister clade to the Thysanoptera (thrips) and the lice (Phthiraptera), making the Hemiptera non-monophyletic, as traditionally understood.
The fossil record of hemipterans goes back to the Moscovian Carboniferous (315 to 307 million years ago). The oldest fossils are of the Archescytinidae from the Lower Permian (299 to 251 million years ago) and are thought to be basal to the Auchenorrhyncha (cicadas, leafhoppers, treehoppers, planthoppers, and spittlebugs). The infraorders Fulguromorpha (planthoppers) and Cicadomorpha (cicadas, leafhoppers, treehoppers, and spittlebugs) appear in the Upper Permian, as do Sternorrhyncha of the superfamilies Psylloidea (jumping plant lice) and Aleurodoidea (whiteflies). The first Heteroptera as well as aphids and coccoids appeared in the Triassic (251 to 201 million years ago). The Suborder Coleorrhyncha (moss or beetle bugs) can be found in the fossil record back to the Lower Jurassic (201 million years ago).
Hemipterans range in size from 1 mm (0.04 in.) to around 15 cm (6 in.), and share a common arrangement of sucking mouthparts. The name “true bugs” is sometimes limited to the suborder Heteroptera (a group of about 40,000 species).
Most hemipterans are phytophagous, using their sucking and piercing mouthparts to feed on plant sap. These include aphids, cicadas, froghoppers, leafhoppers, planthoppers, scale insects, treehoppers, and whiteflies, and some other groups of terrestrial herbivores such as broad-headed bugs (Alydidae), stilt bugs (Berytidae), lace bugs (Tingidae), scentless plant bugs (Rhopalidae), and squash bugs and leaf-footed bugs (Coreidae). Some are monophagous, being host-specific and found on only one plant taxon, while others are oligophagous, feeding on a few plant groups, while others again are much less discriminating and polyphagous, consuming many plants. Other hemipterans are parasites and/or predators that feed on other insects, small invertebrates, and even vertebrates. Interestingly, some members of the family Largidae (bordered plant bugs) resemble ants (Hymenoptera). They live as social parasites in ant nests, mimicking the behavior of ants in order to get food.
Hemipterans are hemimetabolous, meaning that they do not undergo complete metamorphosis. Instead, their young (termed nymphs) resemble miniature adults. The nymph molts several times, and each larval instar resembles the adult a little more than the previous instar. The wing buds grow in later stage nymphs, and the final transformation involves development of functional wings (if they are present at all) and sexual organs, with no principal pupal stage as in holometabolous (complete metamorphic) insects.
Many aphids are parthenogenetic during part of the life cycle, such that females can produce unfertilized eggs without sperm, where the resultant young are basically clones of their mother. All such young are females (termed thelytoky), so that at these times all of the population can produce more offspring. On the other hand, many other species of aphids are also viviparous whereby the young are born live rather than laid as eggs. These adaptations enable aphids to reproduce extremely rapidly when environmental conditions are at their peak.
Humans have interacted with the Hemiptera for centuries. Some species, such as aphids, are significant agricultural pests. They damage crops by the direct action of sucking plant sap, as well as harming them indirectly by being the vectors of serious viral pathogens. Other species have been used for biological control of insect pests. Hemipterans, such as the scale insect (Dactylopius coccus), have been cultivated for the extraction of the brilliant red-colored dyestuff cochineal (also known as carminic acid) and lac (scale) bugs (Kerria laca) for scarlet resinous secretions such as shellac. Interestingly, up to 100,000 scale insects are needed to make one kilogram (2.2 lbs.) of cochineal dye, and a similar number of lac bugs are needed to make one kilogram of shellac, a brush-on colorant and wood finish. Additional uses of this traditional product include the waxing of citrus fruits to extend their shelf-life, and the moisture-proof coating of medicinal pills, providing a slow-release or masking the taste of bitter ingredients.
The mouthpart or “beak” of a typical hemipteran is distinctive, with maxillae and mandibles modified to form a piercing sheathed “stylet” within a modified labium. This stylet, with the supporting labrium, is capable of piercing tissues and sucking liquids and contains a channel for the outward movement of saliva and another for the inward movement of liquid food. A salivary pump powered by substantial dilator muscles in the head drives saliva into a prey item, whereas a cibarial muscular pump extracts liquid from it by contractile action. When not in use, the beak is usually folded under the body. The diet of most hemipterans is typically plant sap, but some, such as assassin bugs (Reduviidae), are blood-suckers, and a few are predators. Both herbivorous and predatory hemipterans inject hydrolysis, depolymerase, and protease enzymes to begin digestion extraorally (before the food is taken into the body).
Although the Hemiptera vary widely in their overall form, their mouthparts form a distinctive “rostrum.” Various other insects can be confused with Hemiptera, but they all have biting mandibles and maxillae instead of the rostrum. These examples include cockroaches (Blattoidea) and psocids (booklice), both of which have longer, many-segmented antennae, and some beetles (Coleoptera), but these have fully hardened forewings that do not overlap.
The forewings of Hemiptera are either entirely membranous, as in the Auchenorrhyncha and Sternorrhyncha, or partially hardened, as in most Heteroptera. The forewings (hemelytra) of many heteropterans are membranous at the ends but hardened or leathery near the base. In all other suborders, the hindwings (if present at all) are entirely membranous and usually shorter than the forewings. The forewings may be held “roofwise” over the body (typical of Auchenorrhyncha and Sternorrhyncha), or held flat on the back, with the ends overlapping (typical of Heteroptera). The tarsi of the legs have two or three segments, while the antennae typically consist of four or five segments.
Many hemipterans produce sound for communication. The “song” of male cicadas, the loudest of any insect, occurs usually in the hottest part of summer and is used to attract mates. It is produced by special corrugated exoskeleton structures (tymbal organs) on the first segment of the abdomen. The tymbals are drum-like disks of alternating stiff and flexible cuticular membranes in the abdomen, which are clicked in and out repeatedly. In addition to attracting females, the male’s song may also serve to attract other males, especially in those species that form noisy mating aggregations. Stridulatory sounds are produced among the aquatic Corixidae (water boatmen) and Notonectidae (backswimmers) using their tibial combs rubbing across rostral ridges.
In many species of Hemiptera, parental care is found, especially in members of the Membracidae (treehoppers) and numerous Heteroptera. Also, in several species of shield or stink bugs (Pentatomidae), females stand guard over their egg clusters to protect them from egg parasitoids and predators. In the aquatic Belostomatidae (giant water bugs), females lay their eggs on the back of the male, which then guards the eggs. Protection provided by ants is common in the Auchenorrhyncha.
Pondskaters (or water striders, Family Gerridae) are adapted to use surface tension to keep above a freshwater surface. Hemipterans make use of a variety of other modes of locomotion, including swimming, jumping, walking, and flying. Several families of Heteroptera are water bugs adapted to an aquatic lifestyle, such as the giant water bugs (Belostomatidae), water boatmen (Corixidae), water striders (Gerridae), water scorpions (Nepidae), creeping water bugs (Naucoridae), and backswimmers (Notonectidae). They are mostly aquatic predators, and some have legs adapted as paddles to help the animal move through the water. Water bugs in the genus Microvelia (Veliidae) can travel at up to 17 cm/second, twice as fast as they can walk on land.
The power of flight is well developed in the Hemiptera, although it is mostly used for short-distance movement and dispersal. Wing development is sometimes related to environmental conditions. For example, in both winged and wingless forms of aphids, winged forms reproduce in greater numbers when food resources are in low supply. Aphids and whiteflies can sometimes be transported very long distances by high-altitude winds and atmospheric updrafts.
Many Auchenorrhyncha (including representatives of the cicadas, leafhoppers, treehoppers, planthoppers, and froghoppers) are adapted for saltation (jumping). Treehoppers, for example, jump by rapidly depressing their hind legs and attaining a take-off velocity of up to 2.7 m/second (8.9 ft./second) and an acceleration of up to 250 g (8.8 oz.). On the other hand, cicadas, the largest hemipterans, extend their hind legs for a jump in under a millisecond. Most female Sternorrhyncha (aphids, whiteflies, and scale insects), instead of relying on any form of locomotion, are sedentary or completely sessile, attached to their host plants by their thin feeding stylets, which cannot be removed from the plant very quickly.
Hemipterans have been reported to dramatically reduce the mass of affected plants, especially in major outbreaks. The life cycle of some species involves an alternation between two species of host plants, for example between an annual crop and a woody plant. Some species feed on only one type of plant, while others are generalists, colonizing many plant groups. They sometimes can also change the mix of plants by predation on seeds or feeding on roots of certain species. Some sap-suckers move from one host to another at different times of the year. Many aphids spend the winter as eggs on a woody host plant and the summer as parthenogenetically reproducing females on herbaceous plants.
Although many hemipterans, including many species of aphid and scale insects, are significant pests of crops and garden plants, other species are harmless. The damage done is often not the withdrawal of the plant sap, but the fact that they transmit serious viral diseases between plants. For example, ash-gray leaf bugs (Piesmatidae, Piesma) are vectors of the beet leafcurl virus, the sugarbeet savoy virus, and beet latent rosette disease. In addition, aphids are known to transmit over 150 different kinds of plant viruses, leafhoppers transmit over eighty known types of plant disease, planthoppers (Superfamily Fulgoroidea) have been implicated as vectors in the transmission of about twenty plant diseases, and whiteflies are responsible for transmitting yellow mosaic diseases in at least twenty plant species.
Some Sternorrhyncha including psyllids (jumping plant lice) and some aphids are gall formers. These sap-sucking hemipterans inject fluids containing plant hormones into the plant tissues, inducing the production of tissue that covers to protect the insect and also acts as sinks for nutrition that they feed on. The hackleberry nipple gall psyllid (Pachypsylla) for example, causes a woody gall on the leaf petioles of the hackleberry tree (Celtis occidentalis) it infests, and the nymph of another psyllid produces a protective lerp (crystallized honeydew) out of hardened honeydew.
Most other hemipterans are predatory, either as nymphs or adults, feeding on insects or snails, or even small vertebrates (tadpoles and fish). For example, the predatory shield bug (Euthyrhynchus floridanus) stabs caterpillars with its beak and sucks out the body fluids. The saliva of predatory heteropterans contains digestive enzymes, and their mouthparts are adapted for predation. There are toothed stylets on the mandibles able to cut into and scrape tissues of their prey. There are further stylets on the maxillae, adapted as tubular canals to inject saliva and to extract the pre-digested and liquefied contents of the prey. Some species attack pest insects and are used in biological control. One of these is the spined soldier bug (Podisus maculiventris) that sucks body fluids from larvae of the Colorado potato beetle (Leptinotarsa decemlineata) and the Mexican bean beetle (Epilachna varivestis).
A few hemipterans are parasitic or hematophagic, feeding on the blood of larger mammals, including humans. These include bedbugs (Cimex spp.) and the triatomine kissing, conenose, or assassin bugs (Rhodnius, Family Reduviidae), which are vectors of the protist Trypanosoma cruzi, which causes Chagas disease in humans in the southwestern United States. They are termed kissing bugs because they suck blood from around the lips while a person sleeps. The bed bug, Cimex lectularius, is a persistent ectoparasite of humans. The common name “bed bug” is used for this bug because it likes to live in houses and is especially active at night in beds or other areas where people may sleep, feeding on human blood, generally without being noticed. Unlike other insects, male bedbugs do not place their sperm directly in the female’s reproductive tract. Instead, they mate by traumatic insemination; the male punctures the female’s abdomen and injects his sperm into a secondary genital structure, the spermalege. The sperm travel in the female’s blood (hemolymph) to sperm storage structures (seminal conceptacles); they are released from there to fertilize her eggs inside her ovaries. Other Cimex spp. occurs on various species of bats, and two species, C. adjunctus and C. pilosellus, have been reported on several bats from Arkansas.
Members of the families Anthocoridae (minute pirate bugs), Phymatidae (ambush bugs), and Nabidae (damsel bugs) are obligate predators. Some predatory species are used in biological pest control; these include various nabids that feed on aphids, and even some members of families that are primarily phytophagous, such as the genus Geocoris (big-eyed bugs) in the family Lygaeidae (seed bugs). The chinch bug (Blissus leucopterus) is most likely the most injurious bug on corn, wheat, and other cereals and may also be a pest of turf grasses. Other hemipterans are omnivores, alternating between a plant-based and an animal-based diet. For example, the mirid, Dicyphus hesperus, is used to control greenhouse whiteflies (Trialeurodes vaporariorum) on tomatoes but also sucks sap and, if deprived of plant tissues, will die even when whiteflies are plentiful.
Insects, in general, have high protein contents and possess good food conversion ratios; however, most hemipterans are too small to be a useful component of the human diet. At least nine species of Hemiptera have been reported to be eaten worldwide.
In terms of conservation, large-scale cultivation of the oil palm (Elaeis guineensis) in the Amazon basin damages freshwater habitats and reduces the diversity of aquatic and semi-aquatic Heteroptera. In addition, climate change may be affecting the global migration of hemipterans such as the potato leafhopper (Empoasca fabae). Global warming appears to be correlated with the severity of E. fabae infestation, so increased warming may exacerbate infestations in the future.
A great deal of information has been gained on the Hemiptera of Arkansas. Much of the work has been done by Arkansas State University (ASU) faculty and the students they mentored. Notable among these are the late Harvey E. Barton, a specialist of pentatomids, and George L. Harp, a hemipteran generalist, who mentored Stephen W. Chordas III, the most prolific writer, to date, on Arkansas Hemiptera.
No hemipterans are currently endangered or threatened in Arkansas. However, the lace bug (Acalypta susanae, Family Tingidae), an endemic originally known only from Mount Magazine (Logan County), is on the list of Species of Greatest Conservation Need. Additional specimens of A. susanae were collected in 2013 near the Buffalo National River (Newton County). The IUCN (International Union for Conservation of Nature) lists two species of Hemiptera as extinct, including the mealybugs, Clavicoccus erinaceus and Phyllococcus oahuensis. Both were endemic to Hawaii, where the former lived on its host plant, the now critically endangered greenflower Indian mallow (Abutilon sandwicense).
For additional information:
Allen, Robert T., Christopher E. Carlton, and S. A. Tedder. “A New Species of Acalypta (Hemiptera, Tingidae) from Arkansas.” Journal of the Kansas Entomological Society 61 (1988): 126‒130.
Barton, Harvey E. “Checklist of Pentatomoidea (Insecta: Hemiptera) of Arkansas.” Arkansas Academy of Science, Arkansas Biota Survey Checklist 46 (1987): 1‒2.
Barton, Harvey E., and Linda A. Lee. “The Pentatomidae of Arkansas USA.” Proceedings of the Arkansas Academy of Science 35 (1981): 20‒25. Online at https://scholarworks.uark.edu/jaas/vol35/iss1/7/ (accessed December 30, 2019).
Chordas, Stephen W., III. “Literature Record Checklist of True Bugs (Hemiptera) for Arkansas, U.S.A., as of 2018.” Journal of the Arkansas Academy of Science 71 (2017): 224‒231. Online at https://scholarworks.uark.edu/jaas/vol71/iss1/42/ (accessed December 30, 2019).
Chordas, Stephen W., III, and Chris T. McAllister. “Eastern Boxelder Bug, Boisea trivittata (Hemiptera: Rhopalidae) Confirmation in Arkansas.” Journal of the Arkansas Academy of Science 69 (2015). Online at https://scholarworks.uark.edu/jaas/vol69/iss1/24/ (accessed December 30, 2019).
Chordas, Stephen W., III, Chris T. McAllister, and Henry W. Robison. “The Introduced Dirt-Colored Seed Bug, Megalonotus sabulicola (Hemiptera: Rhyparochromidae): New for Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 135‒136. Online at https://scholarworks.uark.edu/jaas/vol68/iss1/22/ (accessed December 30, 2019).
Chordas, Stephen W., III, and George L. Harp. “A Synopsis of the Notonectidae of Arkansas.” Proceedings of the Arkansas Academy of Science 45 (1991): 117–119. Online at https://scholarworks.uark.edu/jaas/vol45/iss1/36/ (accessed December 30, 2019).
Chordas, Stephen W., III, Henry W. Robison, Eric G. Chapman, Betty G. Crump, and Peter W. Kovarik. “Fifty-Four State Records of True Bugs (Hemiptera: Heteroptera) from Arkansas.” Journal of the Arkansas Academy of Science 59 (2005): 43‒50. Online at https://scholarworks.uark.edu/jaas/vol59/iss1/7/ (accessed December 30, 2019).
Chordas, Stephen W., III, and Joe Kremers. “Backyard ‘Bug’ Collecting Results in 6 New State Records for Arkansas, U.S.A.” Journal of the Arkansas Academy of Science 63 (2009): 177‒179. Online at https://scholarworks.uark.edu/jaas/vol63/iss1/23/ (accessed December 30, 2019).
———. “Two Lygaeoidea (Hemiptera), Ischnodemus slossonae and Cryphula trimaculata, New for Arkansas, U.S.A.” Journal of the Arkansas Academy of Science 62 (2008): 147–147. Online at https://scholarworks.uark.edu/jaas/vol62/iss1/24/ (accessed December 30, 2019).
Chordas, Stephen W., III, and Peter W. Kovarik. “Two Coreidae (Hemiptera), Chelinidea vittiger and Anasa armigera, New for Arkansas, U.S.A.” Journal of the Arkansas Academy of Science 62 (2008): 145‒146. Online at https://scholarworks.uark.edu/jaas/vol62/iss1/23/ (accessed December 30, 2019).
———. “Two Lygaeoidea (Hemiptera), Ischnodemus slossonae and Cryphula trimaculata, New for Arkansas, U.S.A.” Journal of the Arkansas Academy of Science 62 (2008): 147. Online at https://scholarworks.uark.edu/jaas/vol62/iss1/24/ (accessed December 30, 2019).
Chordas, Stephen W., III, and Renn Tumlison. “Four Uncommon Assassin Bugs (Hemiptera: Reduviidae: Emesinae) New for Arkansas, U.S.A.” Entomological News 126 (2016): 77‒82.
———. “Sex-Ratio of Miridae (Hemiptera) Taken Via UV Light-Traps in Arkansas, USA.” Journal of the Arkansas Academy of Science 71 (2017): 200‒202. Online at https://scholarworks.uark.edu/jaas/vol71/iss1/34/ (accessed December 30, 2019).
Chordas, Stephen W., III, Renn Tumlison, and Chris T. McAllister. “First Report of the True Bug Pseudopachybranchius vinctus (Hemiptera: Rhyparochromidae) from Arkansas and Oklahoma, U.S.A.” Entomological News 127 (2018): 269‒272.
Chordas, Stephen W., III, Renn Tumlison, Henry W. Robison, and Joe Kremers. “Twenty-Three True Bug Records for Arkansas, with Two for Ohio, U.S.A.” Journal of the Arkansas Academy of Science 65 (2011): 153–159. Online at https://scholarworks.uark.edu/jaas/vol65/iss1/22/ (accessed December 30, 2019).
Chordas, Stephen W., III, Renn Tumlison, and Karen Benjamin. “The Genus Reuteria(Hemiptera: Miridae) with Five Species New for Arkansas, U.S.A.” Journal of the Arkansas Academy of Science 67 (2013): 163‒164. Online at https://scholarworks.uark.edu/jaas/vol67/iss1/28/ (accessed December 30, 2019).
Gaspar, J. P., C. R. Minteer, Tonya McKay, and S. Raghu. “First Records for Pseudomops septentrionalis Hebard (Blattodea: Ectobiidae) and Acantholomidea porosa (Germar) (Heteroptera: Scutelleridae), in Arkansas.” Journal of the Kansas Entomological Society 88 (2015): 124‒127.
Goddard, Jerome, and R. deShazo. “Bed Bugs (Cimex lectularius) and Clinical Consequences of Their Bites.” Journal of the American Medical Association 301 (2009): 1358–1366.
Grilliot, M. E., John L. Hunt, C. G. Sims, and C. E. Come. “New Host and Location Record for the Bat Bug Cimex adjunctus Barber, 1939, with a Summary of Previous Records.” Journal of the Arkansas Academy of Science 68 (2014): 149‒151. Online at https://scholarworks.uark.edu/jaas/vol68/iss1/26/ (accessed December 30, 2019).
Harp, George L. “Synopsis of the Hydrometridae of Arkansas.” Proceedings of the Arkansas Academy of Science 39 (1985): 130‒131. Online at https://scholarworks.uark.edu/jaas/vol39/iss1/37/ (accessed December 30, 2019).
———. “A Synopsis of the Nepidae of Arkansas.” Proceedings of the Arkansas Academy of Science 39 (1985): 128‒130. Online at https://scholarworks.uark.edu/jaas/vol39/iss1/36/ (accessed December 30, 2019).
Harp, Phoebe A., and Harvey E. Barton. “Notes on the Biology of Thyanta calceata (Hemiptera: Pentatomidae) on Tephrosia virginiana (Leguminosae), a New Host Plant.” Proceedings of the Arkansas Academy of Science 42 (1988): 110‒111. Online at https://scholarworks.uark.edu/jaas/vol42/iss1/34/ (accessed December 30, 2019).
Harp, Phoebe A., and George L. Harp. “A Synopsis of the Belostomatidae of Arkansas.” Proceedings of the Arkansas Academy of Science 44 (1990): 129‒130. Online at https://scholarworks.uark.edu/jaas/vol44/iss1/36/ (accessed December 30, 2019).
Henry, Thomas J., G. F. Hevel, and Stephen W. Chordas III. “Additional Records of the Little Known Corixidea major (Heteroptera: Schizopteridae) from Arkansas and Oklahoma.” Proceedings of the Entomological Society of Washington 112 (2010): 475‒477.
Hoell, H. V., J. T. Doyen, and A. H. Purcell. Introduction to Insect Biology and Diversity. 2nd ed. London: Oxford University Press, 1998.
Kittle, Paul D. “The Biology of Water Striders (Hemiptera: Gerridae) in Northwest Arkansas.” American Midland Naturalist 97 (1977): 400‒410.
———. “The Water Striders (Hemiptera: Gerridae) of Arkansas.” Proceedings of the Arkansas Academy of Science 34 (1980): 68‒71. Online at https://scholarworks.uark.edu/jaas/vol34/iss1/21/ (accessed December 30, 2019).
Lee, Linda A., and Harvey E. Barton. “Distribution and Seasonal Occurrence of the Scutelleridae, Corimelaenidae and Cydnidae of Arkansas.” Proceedings of the Arkansas Academy of Science 37 (1983): 42‒48. Online at https://scholarworks.uark.edu/jaas/vol37/iss1/13/ (accessed December 30, 2019).
Polhemus, J. T. “A New Species of Pentacora (Heteroptera: Saldidae) from the Ouachita Mountains of Arkansas.” Journal of the Kansas Entomological Society 66 (1993): 455‒457.
Price, Alan D., V. Rick McDaniel, and Renn Tumlison. “An Infestation of the Bat Bug Cimex pilosellus on an Arkansas Population of Big Brown Bats (Eptesicus fuscus).” Proceedings of the Arkansas Academy of Science 36 (1982): 98. Online at https://scholarworks.uark.edu/jaas/vol36/iss1/35/ (accessed December 30, 2019).
Reinhardt, Klaus, and Michael T. Siva-Jothy. “Biology of the Bed Bugs (Cimicidae).” Annual Review of Entomology 52 (2007): 351–374.
Sasse, D. Blake, Chris T. McAllister, and Lance A Durden. “A New Host Record for the Bat Bug, Cimex adjunctus (Insecta: Hemiptera) from Eastern Small-Footed Myotis, Myotis leibii (Chiroptera: Vespertilionidae).” Journal of the Arkansas Academy of Science 70 (2016): 287‒288. Online at https://scholarworks.uark.edu/jaas/vol70/iss1/50/ (accessed December 30, 2019).
Skvarla, Michael J., Danielle M. Fisher, and Ashley P. G. Dowling. “Arthropods of Steel Creek, Buffalo National River, Arkansas. III. Heteroptera (Insecta: Hemiptera).” Biodiversity Data Journal 4 (2016): e7607.
Smith, J. F., R. G. Luttrell, and J. K. Greene. “Seasonal Abundance, Species Composition, and Population Dynamics of Stink Bugs in Production Fields of Early and Late Soybean in South Arkansas.” Journal of Economic Entomology 102 (2009): 229‒236.
Snodgrass, G. L., Thomas J. Henry, and W. P. Scott. “An Annotated List of the Miridae (Heteroptera) Found in the Yazoo-Mississippi Delta and Associated Areas in Arkansas and Louisiana.” Proceedings of the Entomological Society of Washington 86 (1984): 845‒860.
Snodgrass, G. L., W. P. Scott, and J. W. Smith. “An Annotated List of the Host Plants of Lygus lineolaris (Hemiptera: Miridae) in the Arkansas, Louisiana & Mississippi Delta.” Journal of the Georgia Entomological Society 19 (1984): 93‒101.
———. “Host Plants and Seasonal Distribution of the Tarnished Plant Bug (Hemiptera: Miridae) in the Delta of Arkansas, Louisiana and Mississippi.” Environmental Entomology 13 (1984): 110‒116.
———. “Host Plants of Taylorilygus pallidulus and Polymerus basalis (Hemiptera: Miridae) in the Delta of Arkansas, Louisiana, and Mississippi. Florida Entomologist 67 (1984): 402‒408.
———. “A Survey of the Host Plants and Seasonal Distribution of the Cotton Fleahopper (Hemiptera: Miridae) in the Delta of Arkansas, Louisiana, and Mississippi.” Journal of the Georgia Entomological Society 19 (1984): 34‒41.
Steward, Tim W., V. Rick McDaniel, and Daniel R. England. “Additional Records of Distribution and Hosts for the Bat Bug, Cimex pilosellus, in Arkansas.” Proceedings of the Arkansas Academy of Science 40 (1986): 95‒96. Online at https://scholarworks.uark.edu/jaas/vol40/iss1/36/ (accessed December 30, 2019).
Taylor, S. J., and J. E. McPherson. “State Records and Confirmations of Arkansas Flat Bugs (Heteroptera: Aradidae).” Great Lakes Entomologist 22 (1989): 19‒23.
Tumlison, Renn, and Stephen W. Chordas III. “Three Uncommon Nabidae (Hemiptera), Alloeorhynchus trimacula, Carthasis decoratus and Phorticus collaris New for Arkansas, USA.” Entomological News 128 (2018): 49‒52.
Chris T. McAllister
Eastern Oklahoma State College
Henry W. Robison
Sherwood, Arkansas
 Science and Technology
Science and Technology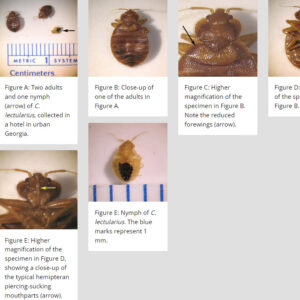 Bedbugs
Bedbugs  Hemiptera (a.k.a. True Bugs)
Hemiptera (a.k.a. True Bugs)  Kissing Bug
Kissing Bug 




Comments
No comments on this entry yet.